Gut Microbiota-Derived Acetate Ameliorates Endometriosis via JAK1/STAT3-Mediated M1 Macrophage Polarisation
- PMID: 40739711
- PMCID: PMC12310558
- DOI: 10.1111/1751-7915.70202
Gut Microbiota-Derived Acetate Ameliorates Endometriosis via JAK1/STAT3-Mediated M1 Macrophage Polarisation
Abstract
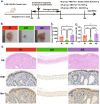
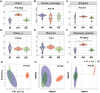
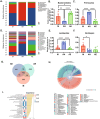
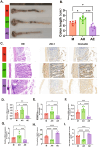
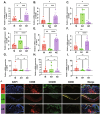
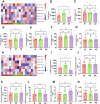
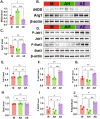
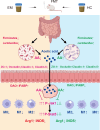
Endometriosis (EMs) is a common inflammatory disorder in women of reproductive age, severely impacting patients' quality of life and fertility. Current hormonal therapies offer limited efficacy, and surgical interventions often fail to prevent recurrence. Recent studies suggest a close association between gut microbiota and the pathophysiology of EMs, though the precise mechanisms remain unclear. To investigate the influence of gut microbiota on EMs, this study established an EMs mouse model and performed faecal microbiota transplantation (FMT) using samples from healthy donors (AH group) and EMs patients (AE group) into the model mice. Results demonstrated that compared to the model group (M group), FMT from healthy donors (AH group) significantly reduced ectopic lesion volume (658.3 ± 116.1 vs. 167.2 ± 112.8 mm3, p < 0.01) and weight (0.7420 ± 0.1233 vs. 0.1885 ± 0.1239 mg, p < 0.01). Conversely, FMT from EMs patients exacerbated disease progression. Mechanistic studies revealed that healthy donor FMT attenuated EMs by remodelling the gut microbial composition (enhancing α-diversity and Lactobacillus abundance while suppressing Bacteroidetes), significantly elevating acetate levels in faeces and ectopic lesions, activating the JAK1/STAT3 signalling pathway within lesions, and thereby driving macrophage polarisation toward the M1 phenotype (by increased iNOS/CD86 expression and decreased Arg1/CD206 expression). Simultaneously, healthy donor FMT enhanced intestinal barrier integrity by upregulating tight junction proteins (ZO-1, Occludin, Claudin-1/5) and reducing levels of intestinal permeability markers (DAO, IFABP). In contrast, AE group FMT disrupted gut microbial ecology, reduced acetate production, failed to activate the JAK1/STAT3 pathway, promoted M2 macrophage polarisation and impaired intestinal barrier function. Collectively, this study elucidates for the first time that acetate, as a key gut microbiota metabolite, exerts anti-EMs effects by activating the JAK1/STAT3 signalling pathway to drive macrophage reprogramming toward the M1 phenotype, thereby positioning gut microbiota reconstruction as a novel therapeutic strategy for endometriosis.
Keywords: JAK–STAT signalling; acetate; endometriosis; faecal microbiota transplantation; gut microbiota; macrophage polarisation; short‐chain fatty acids (SCFAs).
© 2025 The Author(s). Microbial Biotechnology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest. The (partial) results of the present study were orally presented in the China Gut Conference 2025 (Ningbo).
Figures
Similar articles
-
Intestinal inflammation and microbiota modulation impact cochlear function: emerging insights in gut-ear axis.Cell Commun Signal. 2025 Jul 26;23(1):357. doi: 10.1186/s12964-025-02338-1. Cell Commun Signal. 2025. PMID: 40713718 Free PMC article.
-
Milk-processed Polygonatum cyrtonema Hua ameliorates cyclophosphamide-induced immunosuppression in mice by regulating gut microbiota and immune response.Phytomedicine. 2025 Sep;145:157076. doi: 10.1016/j.phymed.2025.157076. Epub 2025 Jul 14. Phytomedicine. 2025. PMID: 40684494
-
Donor-derived microbial engraftment and gut microbiota shifts associated with weight loss following fecal microbiota transplantation.Appl Environ Microbiol. 2025 Jul 23;91(7):e0012025. doi: 10.1128/aem.00120-25. Epub 2025 Jun 4. Appl Environ Microbiol. 2025. PMID: 40464558 Free PMC article. Clinical Trial.
-
Gut microbiome-based interventions for the management of obesity in children and adolescents aged up to 19 years.Cochrane Database Syst Rev. 2025 Jul 10;7(7):CD015875. doi: 10.1002/14651858.CD015875. Cochrane Database Syst Rev. 2025. PMID: 40637175 Review.
-
Exploring the role of gut microbiota in Parkinson's disease: insights from fecal microbiota transplantation.Front Neurosci. 2025 Jun 13;19:1574512. doi: 10.3389/fnins.2025.1574512. eCollection 2025. Front Neurosci. 2025. PMID: 40584885 Free PMC article. Review.
References
-
- Agarwal, S. K. , Chapron C., Giudice L. C., et al. 2019. “Clinical Diagnosis of Endometriosis: A Call to Action.” American Journal of Obstetrics and Gynecology 220, no. 4: 354.e1–354.e12. - PubMed
-
- Bedaiwy, M. A. , Allaire C., and Alfaraj S.. 2017. “Long‐Term Medical Management of Endometriosis With Dienogest and With a Gonadotropin‐Releasing Hormone Agonist and Add‐Back Hormone Therapy.” Fertility and Sterility 107, no. 3: 537–548. - PubMed
-
- Berni, C. R. 2019. “Lactobacillus for Gastroenteritis in Children.” New England Journal of Medicine 380, no. 19: e36. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

