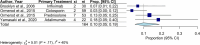Treatments for Pyoderma Gangrenosum: A Systematic Review and Single-Arm Meta-Analysis of Systemic Therapies
- PMID: 40740034
- PMCID: PMC12311392
- DOI: 10.1111/iwj.70733
Treatments for Pyoderma Gangrenosum: A Systematic Review and Single-Arm Meta-Analysis of Systemic Therapies
Abstract
Pyoderma gangrenosum (PG) is a neutrophilic dermatosis associated with significant morbidity and mortality, with no consensus treatment to date. To review all clinical trials of treatments for PG to synthesise clinical evidence regarding the efficacy and safety of different treatments. After PROSPERO (CRD42023459180) registration, we systematically searched five databases (clinicaltrials.gov, CENTRAL, Embase, PubMed and Scopus) up until 18th May 2024 for PG treatments. Of 10 579 identified articles, 5853 deduplicated abstracts were screened. Twenty studies met the screening criteria after a full text review of 60 articles. We assessed the risk of bias using ROBIN-I for non-randomised and ROB-2 for randomised trials. Two reviewers independently performed article screening and quality assessments. Two reviewers independently extracted and recorded data on study characteristics, participants' demographics, disease characteristics, treatment regimens, and outcomes for the selected studies. A single-arm meta-analysis of available RCTs and non-randomised studies was conducted to analyse the outcomes of different systemic immunomodulators. The primary outcome was the complete healing of PG. Secondary outcomes included rates of recurrence, treatment failure, adverse events and time to complete healing. A total of twenty (20) interventional studies were included in the data synthesis: nine (9) prospective open-label studies, six (6) prospective cohort studies, three (3) open-label clinical trials, and two (2) randomised controlled trials evaluating multiple biological, systemic, and topical interventions. On random effects meta-analysis of systemic therapies including adalimumab, canakinumab, infliximab, chlorambucil, cyclosporine, cyclophosphamide and prednisolone, the pooled proportion of complete healing across 11 studies was 0.59 (95% confidence interval [CI]: 0.41-0.74; Χ2 = 26.66, p < 0.01; I2 = 66%); the pooled proportion of PG recurrence across 6 studies was 0.30 (95% CI: 0.20-0.41; Χ2 = 1.14, p = 0.95; I2 = 0%); the pooled proportion of serious adverse effects from 4 studies was 0.10 (95% CI: 0.05-0.19; Χ2 = 5.01, p = 0.17; I2 = 40%); and the pooled proportion of PG treatment failure across seven studies was 0.36 (95% CI: 0.24-0.49; Χ2 = 12.78, p = 0.03; I2 = 61%). The proportion of complete wound healing varies significantly across treatments and recurrence is common even in a limited follow-up period. Heterogeneity of study methods and low numbers hamper disease research. There remains a significant unmet need for better outcome measures than just complete healing as well as better treatment options to improve patient outcomes.
Keywords: clinical trials; efficacy; pyoderma gangrenosum; safety; skin ulcer.
© 2025 The Author(s). International Wound Journal published by Medicalhelplines.com Inc and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Barbe M., Batra A., Golding S., et al., “Pyoderma Gangrenosum: A Literature Review,” Clinics in Podiatric Medicine and Surgery 38, no. 4 (2021): 577–588. - PubMed
-
- Kridin K., Cohen A. D., and Amber K. T., “Underlying Systemic Diseases in Pyoderma Gangrenosum: A Systematic Review and Meta‐Analysis,” American Journal of Clinical Dermatology 19, no. 4 (2018): 479–487. - PubMed
-
- DeFilippis E. M., Feldman S. R., and Huang W. W., “The Genetics of Pyoderma Gangrenosum and Implications for Treatment: A Systematic Review,” British Journal of Dermatology 172, no. 6 (2015): 1487–1497. - PubMed
-
- Braswell S. F., Kostopoulos T. C., and Ortega‐Loayza A. G., “Pathophysiology of Pyoderma Gangrenosum (PG): An Updated Review,” Journal of the American Academy of Dermatology 73, no. 4 (2015): 691–698. - PubMed
-
- Maverakis E., Ma C., Shinkai K., et al., “Diagnostic Criteria of Ulcerative Pyoderma Gangrenosum: A Delphi Consensus of International Experts,” JAMA Dermatology 154, no. 4 (2018): 461–466. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous