Risk of clinical events in virologically suppressed people with HIV switching to a two-drug regimen vs. remaining on a three-drug regimen: a target trial emulation
- PMID: 40740293
- PMCID: PMC12309018
- DOI: 10.1016/j.eclinm.2025.103368
Risk of clinical events in virologically suppressed people with HIV switching to a two-drug regimen vs. remaining on a three-drug regimen: a target trial emulation
Abstract
Background: Guidelines support the switch to a two-drug regimen (2DR) in virologically suppressed people with HIV (PWH) on a three-drug regimen (3DR). Randomized clinical trials have not included clinical outcomes in study endpoints. We provide estimates of 3-year clinical risk by means of a target trial emulation using the data of a large cohort of PWH in Italy.
Methods: PWH from the Icona Foundation Study who were virologically suppressed (HIV-RNA ≤50 copies/mL) for ≥6 months on a 3DR on or after November 2014, were enrolled (database closure on July 31, 2024). PWH were classified according to therapeutic strategies: switching to 2DR (protease inhibitors or dolutegravir plus lamivudine or dolutegravir plus rilpivirine) or remaining on 3DR (any combination). The primary endpoint was the time to the first clinical composite event (cardiovascular disease [CVD], cancer [AIDS and non-AIDS related], or death). We calculated the difference in 3-year risk between therapeutic strategies, estimated using a weighted non-parametric Kaplan-Meier estimator.
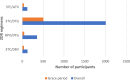
Findings: 7672 participants entered the analysis: 629 (8.2%) switching to 2DR and 7043 (91.8%) remaining on 3DR. Over the 3-year follow-up, 408 events were registered (64 CVD, 234 cancer, and 110 deaths). The 3-year adjusted risk estimate was 2.55 (95% CI 1.72, 5.33) in 2DR vs. 4.69 (95% CI 4.48, 6.17) in 3DR. The difference (-2.15% [95% CI -3.56%, -0.20%]) in favor of 2DR was mainly driven by events of non-AIDS related cancer and mortality.
Interpretation: This study provides evidence that virologically suppressed PWH can be safely switched to 2DR, and may slightly reduce the 3-year risk of a composite clinical outcome.
Funding: The Icona Foundation Study is supported by unrestricted grants from Gilead Sciences, ViiV Healthcare, Merck Sharpe & Dohme.
Keywords: Antiretroviral therapy; Clinical outcomes; HIV; Two drug regimens.
© 2025 The Authors.
Conflict of interest statement
CM has received research grants from Gilead Sciences, Speaker honoraria from Gilead Sciences, ViiV Healthcare, MSD, Johnson & Johnson, travel grants from Gilead; AGiacomelli received consultancy fees from ViiV Healthcare, Gilead Sciences, MSD and Jannsen travel grant from Gilead Sciences; EQR received travel grants from Gilead Sciences and ViiV Healthcare; AV received consultacy fees from ViiV Healthcare, Gilead Sciences and Astrazeneca; ADV received consultancy fees from ViiV Healthcare; ACostantini has received consultancy fees from Gilead Sciences; ADB received speakers' honoraria from Gilead Sciences, ViiV Healthcare and Janssen-Cilag, has been an advisor for ViiV Healthcare, Gilead Sciences, travel reimbursement by Gilead Sciences, and has received grant for research from Gilead Sciences and ViiV healthcare; GDE received Speaker honoraria and has been an advisor for Gilead Sciences, MSD, AbbVie, Janssen-Cilag and ViiV Healthcare, and research grant from GSK and ViiV Healthcare; VMazzotta received institutional research grant from Gilead Science, speaking honoraria for congress from ViiV Healthcare and consultation fees for ViiV Healtcare, Pfizer, and Gilead Science; A. Castagna received fees from ViiV Healthcare, Gilead Sciences, MSD and Janssen-Cilag; AA served as a paid consultant to Astra Zeneca, Bavarian Nordic, Gilead Sciences, GSK, Janssen-Cilag, MSD, Moderna, Pfizer, and ViiV Healthcare and received institutional research grants from Astra Zeneca, Gilead Sciences and ViiV Healthcare; ACL declared research grant or contract for his institute (UCL) by Icona Foundation, and by European Union's Horizon2020: Grant Agreement No 101046016 “EuCARE: European Cohorts of Patients and Schools to Advance Response to Epidemics” and grant Agreement No 101194735 “VIROMARKERS Consortium Agreement”; AG, VMalagnino AdM, JC, AT have nothing to disclose.
Figures
References
-
- European AIDS Clinical Society (EACS) guidelines. 2024. http://www.eacsociety.org Available at: v12.1.
-
- Gandhi R.T., Landovitz R.J., Sax P.E., et al. Antiretroviral drugs for treatment and prevention of HIV in adults: 2024 recommendations of the international antiviral society–USA panel. JAMA. 2025;333:609–628. - PubMed
-
- Panel on Antiretroviral Guidelines for Adults and Adolescents Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Department of health and human services. https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult... Available at:
-
- De Wit S., Bonnet F., Osiyemi O., et al. Durable efficacy of switching from a 3- or 4-drug Tenofovir Alafenamide-Based regimen to the 2-drug regimen Dolutegravir/lamivudine in the TANGO study through week 196. J Acquir Immune Defic Syndr. 2024;96:156–160. - PubMed
-
- Llibre J.M., Brites C., Cheng C.Y., et al. Efficacy and safety of switching to the 2-drug regimen Dolutegravir/Lamivudine versus continuing a 3- or 4-drug regimen for maintaining Virologic suppression in adults living with Human Immunodeficiency Virus 1 (HIV-1): week 48 results from the phase 3, non-inferiority SALSA randomized trial. Clin Infect Dis. 2023;76:720–729. - PMC - PubMed