Fear, facts, and the future: An update on coronavirus disease 2019 vaccine-induced anaphylaxis and vaccine hesitancy among those living with allergy
- PMID: 40740414
- PMCID: PMC12309611
- DOI: 10.1016/j.jacig.2025.100522
Fear, facts, and the future: An update on coronavirus disease 2019 vaccine-induced anaphylaxis and vaccine hesitancy among those living with allergy
Abstract
Background: Despite the low incidence of coronavirus disease 2019 (COVID-19) vaccine-induced allergic reactions reported to date, concerns of such reactions have been reported in the literature among individuals with and without a history of allergic disease.
Objectives: Herein, we provide an update to a previous scoping review published by our group, focusing on COVID-19 vaccine hesitancy in relation to allergy and the incidence of anaphylactic reactions to COVID-19 vaccines.
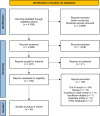
Methods: The current review follows an a priori protocol drafted in accordance with Arksey and O'Malley's framework for methodological reviews. A comprehensive search was conducted on 4 scientific databases (CINAHL, PsycINFO, MEDLINE, Embase) to identify eligible studies published since our initial review. Eligible articles included those published between February 12, 2022, and November 10, 2023, and were retrieved using an established search process developed by content and methodological experts. Among the 2,099 unique citations, 45 articles (2.1%) were included.
Results: Consistent with previously reviewed literature, COVID-19 vaccine-induced anaphylaxis remains rare among both those with allergies and the general population. Despite the rarity of anaphylaxis, hesitancy persists among individuals with and without allergies.
Conclusions: To prepare for future pandemics, it is evident that more efforts are needed to address concerns regarding the potential for allergic reactions following vaccination. As part of this process, it is important to ensure medical professionals are updated as new information becomes available and that evidence-based risk communication is accurate, accessible, and culturally appropriate.
Keywords: Allergy; COVID-19; anaphylaxis; scoping review; vaccine hesitancy.
© 2025 The Authors.
Conflict of interest statement
Funding for this study was provided by a COVID-19 Vaccine Confidence Operating Grant provided by the 10.13039/501100000024Canadian Institutes of Health Research, awarded to J.L.P. The funding body had no influence on the study design, data collection, analysis and interpretation, or manuscript writing. Disclosure of potential conflict of interest: A. L. R. Batac serves on the Manitoba and Saskatchewan Steering Committee for ImmUnity Canada. P. Bégin is a member of Food Allergy Canada’s Healthcare Advisory Board and reports personal fees from ALK-Abelló, Aralez Pharmaceuticals, AstraZeneca, Bausch Health, Novartis, Pfizer, Sanofi-Genzyme, and Valeo Pharma; and has received grants from DBV Technologies, Novartis, Sanofi, and Regeneron Pharmaceuticals. M. Ben-Shoshan serves on several advisory boards, including Food Allergy Canada, Novartis, Sanofi, and Stallergenes Greer; reports personal fees from Bausch Health, Novartis, Sanofi, and Stallergenes Greer; has received grants from Stallergenes Greer; and has participated in clinical trials conducted by Aimmune Therapeutics, Novartis, and Sanofi. E. Ladouceur is the co-founder and director of Science for All Audiences; and reports consultancy for Novartis. J. L. Protudjer is the Section Head for Allied Health, Co-Lead of the Research Pillar for the Canadian Society of Allergy and Clinical Immunology; a member of the steering committee for Canada's National Food Allergy Action Plan; and reports consultancy work for Ajinomoto Cambrooke, ALK-Abelló, Novartis, and Nutricia. The rest of the authors declare that they have no relevant conflicts of interest.
Figures
References
-
- Managing the COVID-19 infodemic: promoting healthy behaviours and mitigating the harm from misinformation and disinformation [Internet]. World Health Organization. https://www.who.int/news/item/23-09-2020-managing-the-covid-19-infodemic... Available at: . Accessed October 7, 2023.
-
- Nilsson L., Brockow K., Alm J., Cardona V., Caubet J.C., Gomes E., et al. Vaccination and allergy: EAACI position paper, practical aspects. Pediatr Allergy Immunol. 2017;28:628–640. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials