Longitudinal effects of sex differences and apolipoprotein E genotype on white matter engagement among elderly
- PMID: 40740432
- PMCID: PMC12308282
- DOI: 10.1093/braincomms/fcaf278
Longitudinal effects of sex differences and apolipoprotein E genotype on white matter engagement among elderly
Abstract
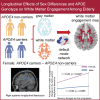
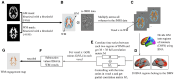
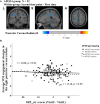
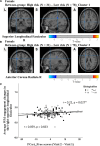
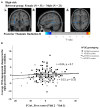
The apolipoprotein E (APOE) ɛ4 allele is the primary genetic risk factor that influences lipid metabolism and contributes to distinctive Alzheimer's disease pathologies, including increased hippocampal atrophy and accelerated cognitive decline. Synaptic dysfunction can occur in APOE4 carriers even before the appearance of any clinical symptoms. Recent evidence has suggested that this genetic risk factor impacts males and females differently. The sex-specific vulnerability for females to cognitive decline, particularly memory, intensifies post-menopause and emphasizes the need for further investigation. White matter abnormalities, APOE4 allele and disruptions in default mode network connectivity serve as early indicators that are crucial for better understanding Alzheimer's disease progression. This study aims to explore relationships between biological sex, APOE4, default mode network-white matter activity and memory function as measured by the Selective Reminding Test. Participants were categorized by risk level on their APOE4 status. Using longitudinal data from the Harvard Aging Brain Study, we examined sex differences in default mode network-white matter engagement among older individuals with and without the APOE4 allele. Our findings demonstrated a significant reduction in default mode network-white matter activity in the right posterior corona radiata in the high-risk group compared to the low-risk group. High-risk females showed reduction in default mode network-white matter activity in the right superior longitudinal fasciculus, which positively correlated with free recall performance, compared to their low-risk counterparts. Unlike females, males showed no significant changes between the low- and high-risk groups. These results underscore the effectiveness of white matter engagement mapping in differentiating longitudinal changes in memory function related to the genetic risk factor APOE4 and biological sex.
Keywords: APOE4; default mode network; memory; sex difference; white matter engagement maps.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report no competing interests.
Figures
Similar articles
-
Time Course and Severity of Cognitive Changes as a Function of Aβ Positivity and APOE Genotype in Alzheimer Disease.Neurology. 2025 Jul 22;105(2):e213853. doi: 10.1212/WNL.0000000000213853. Epub 2025 Jun 27. Neurology. 2025. PMID: 40577676
-
Vascular Dysfunction Is Central to Alzheimer's Disease Pathogenesis in APOE e4 Carriers.Int J Mol Sci. 2022 Jun 26;23(13):7106. doi: 10.3390/ijms23137106. Int J Mol Sci. 2022. PMID: 35806110 Free PMC article.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Behavioral interventions to reduce risk for sexual transmission of HIV among men who have sex with men.Cochrane Database Syst Rev. 2008 Jul 16;(3):CD001230. doi: 10.1002/14651858.CD001230.pub2. Cochrane Database Syst Rev. 2008. PMID: 18646068
References
-
- Navaneetham K, Arunachalam D. Global population aging, 1950–2050. Handbook of aging, health and public policy: Perspectives from Asia. Springer; 2023:1–18.
-
- Fortea J, Pegueroles J, Alcolea D, et al. APOE4 homozygozity represents a distinct genetic form of Alzheimer’s disease. Nat Med. 2024;30(1):1–8. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
