CSF and blood neuronal injury biomarkers in spinal bulbar muscular atrophy and amyotrophic lateral sclerosis 4
- PMID: 40740433
- PMCID: PMC12308280
- DOI: 10.1093/braincomms/fcaf275
CSF and blood neuronal injury biomarkers in spinal bulbar muscular atrophy and amyotrophic lateral sclerosis 4
Abstract
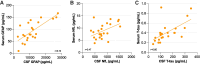
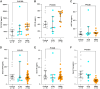
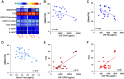
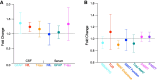
Spinal and bulbar muscular atrophy (SBMA) and amyotrophic lateral sclerosis 4 (ALS4) are two forms of motor neuron disease characterized by clinically slow disease progression. Based on the current limited human studies, the contribution of central nervous neurodegeneration to these diseases and the rate of clinical progression is unclear. Neuronal proteins glial fibrillary acidic protein (GFAP), neurofilament light (NfL) chain, or Total-tau measured in either cerebrospinal fluid or blood could serve as sensitive markers of neurodegeneration. We studied 56 adult participants (32 SBMA, 7 ALS4, and 17 controls) who were enrolled at the National Institutes of Health, of whom 22 (10 SBMA, 7 ALS4, and 5 controls) underwent paired CSF and serum sampling, and of whom 6 participants were assessed longitudinally up to 24 months from initial visit. An additional 7 controls completed CSF sampling only. CSF GFAP, NfL chain, and Total-tau correlated with corresponding levels in serum (r = 0.74, r = 0.47, and r = 0.70, respectively). CSF GFAP was increased in patients with SBMA (median, 8840 pg/mL, interquartile range (IQR) 5780-10489) as compared to controls (median, 5315 pg/mL, IQR 1822-6657; P = 0.029) but not compared with ALS4 (median, 5015 pg/mL, IQR 3172-9803; P = 0.31). Patients with SBMA had increased concentrations of CSF NfL chain (median, 719 pg/mL, IQR 483-773) as compared to ALS4 (median, 307 pg/mL, IQR 187-629; P = 0.034) or controls (median, 395 pg/mL, IQR 307-497; P = 0.024). In contrast, serum concentrations of either biomarker did not differ significantly between SBMA, ALS4, or controls. Higher CSF GFAP and NfL chain levels were associated with lower SBMA Functional Rating Scale scores (r = -0.49 and r = -0.42, respectively). Over the course of 24 months, the average change in SBMA Functional Rating Scale was -0.83 points, while the changes in CSF GFAP and NfL chain were progressive (increased 1.4-fold and 1.3-fold, respectively). Our data suggest that SBMA patients have increased concentrations of CSF GFAP and NfL chain as compared to ALS4 and controls, and higher levels of these biomarkers are associated with disease severity. Importantly, these results indicate that SBMA is associated with progressive neurodegeneration and that either CSF GFAP or NfL chain may be useful for patient stratification and monitoring treatment effects in clinical trials.
Keywords: ALS4; CSF; GFAP; NfL; SBMA.
Published by Oxford University Press on behalf of the Guarantors of Brain 2025.
Conflict of interest statement
The authors report no competing interests.
Figures

Similar articles
-
Serum GFAP predicts survival in advanced ALS: a prospective multicenter study.J Neurol. 2025 Jul 24;272(8):532. doi: 10.1007/s00415-025-13272-0. J Neurol. 2025. PMID: 40705135
-
Association between serum GFAP, BDNF and NfL levels and clinical symptoms in restless legs syndrome: a case-control study.Sleep Med. 2025 Oct;134:106694. doi: 10.1016/j.sleep.2025.106694. Epub 2025 Jul 17. Sleep Med. 2025. PMID: 40706400
-
The impact of kidney function on Alzheimer's disease blood biomarkers: implications for predicting amyloid-β positivity.Alzheimers Res Ther. 2025 Feb 19;17(1):48. doi: 10.1186/s13195-025-01692-z. Alzheimers Res Ther. 2025. PMID: 39972340 Free PMC article.
-
CSF tau and the CSF tau/ABeta ratio for the diagnosis of Alzheimer's disease dementia and other dementias in people with mild cognitive impairment (MCI).Cochrane Database Syst Rev. 2017 Mar 22;3(3):CD010803. doi: 10.1002/14651858.CD010803.pub2. Cochrane Database Syst Rev. 2017. PMID: 28328043 Free PMC article.
-
Treatment for sialorrhea (excessive saliva) in people with motor neuron disease/amyotrophic lateral sclerosis.Cochrane Database Syst Rev. 2022 May 20;5(5):CD006981. doi: 10.1002/14651858.CD006981.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593746 Free PMC article.
References
-
- Kennedy WR, Alter M, Sung JH. Progressive proximal spinal and bulbar muscular atrophy of late onset. A sex-linked recessive trait. Neurology. 1968;18(7):671–680. - PubMed
-
- Sahashi K, Katsuno M, Hung G, et al. Silencing neuronal mutant androgen receptor in a mouse model of spinal and bulbar muscular atrophy. Hum Mol Genet. 2015;24(21):5985–5994. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
