This is a preprint.
Differential Crosslinking and Contractile Motors Drive Nuclear Chromatin Compaction
- PMID: 40740516
- PMCID: PMC12310140
Differential Crosslinking and Contractile Motors Drive Nuclear Chromatin Compaction
Abstract
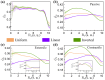
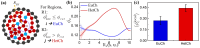
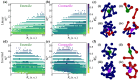
During interphase, a typical cell nucleus features spatial compartmentalization of transcriptionally active euchromatin and repressed heterochromatin domains. In conventional nuclear organization, euchromatin predominantly occupies the nuclear interior, while heterochromatin, which is approximately 50% more dense than euchromatin, is positioned near the nuclear periphery. Peripheral chromatin organization can be further modulated by the nuclear lamina, which is itself a deformable structure. While a number of biophysical mechanisms for compartmentalization within rigid nuclei have been explored, we study a chromatin model consisting of an active, crosslinked polymer tethered to a deformable, polymeric lamina shell. Contractile motors, the deformability of the shell, and the spatial distribution of crosslinks all play pivotal roles in this compartmentalization. We find that a radial crosslink density distribution, even with a small linear differential of higher crosslinking density at the edge of the nucleus, combined with contractile motor activity, drives genomic segregation, in agreement with experimental observations. This arises from contractile motors preferentially drawing crosslinks into their vicinity at the nuclear periphery, forming high-density domains that promote heterochromatin formation. We also find an increased stiffness of nuclear wrinkles given the preferential heterochromatin compaction below the lamina shell, which is consistent with instantaneous nuclear stiffening under applied nanoindentation. We conclude with the potential for experimental validation of our model predictions.
Figures




Similar articles
-
Differential Crosslinking and Contractile Motors Drive Nuclear Chromatin Compaction.bioRxiv [Preprint]. 2025 Jul 27:2025.07.24.666416. doi: 10.1101/2025.07.24.666416. bioRxiv. 2025. PMID: 40777249 Free PMC article. Preprint.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
References
-
- Solovei I., Thanisch K., and Feodorova Y., How to rule the nucleus: divide et impera, Curr. Opin. Cell Biol. 40, 47 (2016). - PubMed
-
- Solovei I., Kreysing M., Lanctôt C., Kösem S., Peichl L., Cremer T., Guck J., and Joffe B., Nuclear architecture of rod photoreceptor cells adapts to vision in mammalian evolution, Cell 137, 356 (2009). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
