Recombinant human thrombopoietin safety and efficacy in pediatric allogeneic hematopoietic stem cell transplantation: A cohort study
- PMID: 40740531
- PMCID: PMC12305145
- DOI: 10.4252/wjsc.v17.i7.106579
Recombinant human thrombopoietin safety and efficacy in pediatric allogeneic hematopoietic stem cell transplantation: A cohort study
Abstract
Background: The safety and efficacy of recombinant human thrombopoietin (rhTPO) administered after allogeneic hematopoietic stem cell transplantation (allo-HSCT) in children (0-9 years old) and adolescents (10-17 years old) with hematological disorders remain unclear.
Aim: To evaluate the safety and efficacy of rhTPO administered before platelet (PLT) engraftment in pediatric patients with hematological disorders undergoing HSCT, and to investigate its effects on the incidence of graft-vs-host disease (GVHD) and other transplant-related outcomes.
Methods: This study enrolled 79 pediatric patients with hematological disorders who received rhTPO after allo-HSCT. The safety and tolerability of rhTPO were evaluated and compared in children (n = 36) and adolescents (n = 43) with hematological disorders. We also investigated the effects of rhTPO administration on the incidence of GVHD and other transplant-related outcomes. Additionally, we examined the efficacy of rhTPO after allo-HSCT in children and adolescents.
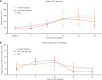
Results: All of the children and adolescents underwent hematopoietic reconstruction. The median time to PLT engraftment was 16 days for all patients, with 14 (range, 11-24) days in the 0- to 9-year-old group and 16 (range, 11-41) days in the 10- to 17-year-old group; the difference was statistically significant (P < 0.05). The median time to neutrophil engraftment was 12 days in both groups. The median recovery times for PLT counts of ≥ 20 × 109/L and ≥ 50 × 109/L in the 0- to 9-year-old group were 10 (range, 2-20) and 11 (range, 2-20) days, respectively, and those for the 10- to 17-year-old group were 9 (range, 4-23) and 12 (range, 5-34) days, respectively. Children exhibited significantly shorter time to PLT engraftment (14 days vs 16 days) and shorter recovery time to PLT count ≥ 100 × 109/L (16 days vs 18 days) (P < 0.05) than adolescents. The incidence of acute GVHD in all patients was 53.2%, with a higher incidence in children (61.1%) than in adolescents (46.5%). The incidence of chronic GVHD showed little difference between the two age groups, with an overall incidence of 10.1%. No adverse events, other than bleeding, were observed in either age group. The incidence of bleeding was 20.3%. The median follow-up time for all survivors was 573 days (range: 42-1803 days) after transplantation. At the final follow-up, 3 patients in the 0- to 9-year-old group died; however, none of these deaths were attributed to allo-HSCT or the use of rhTPO. All patients survived in the 10- to 17-year-old group.
Conclusion: rhTPO was not associated with any significant safety issues and was well tolerated by pediatric and adolescent patients with hematologic diseases who underwent allo-HSCT. Our results suggested that rhTPO may benefit allo-HSCT in children and adolescents by improving PLT recovery.
Keywords: Allogeneic hematopoietic stem cell transplantation; Graft-vs-host disease; Pediatric hematologic disorders; Platelet engraftment; Recombinant human thrombopoietin.
©The Author(s) 2025. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors have no conflicts of interest to declare.
Figures
Similar articles
-
Herombopag Versus rhTPO in Stem Cell Transplantation: A Comparative Study of Efficacy, Safety, and Cost-Effectiveness.Transplant Cell Ther. 2025 Jun 28:S2666-6367(25)01266-7. doi: 10.1016/j.jtct.2025.06.023. Online ahead of print. Transplant Cell Ther. 2025. PMID: 40588097
-
Bone marrow versus peripheral blood allogeneic haematopoietic stem cell transplantation for haematological malignancies in adults.Cochrane Database Syst Rev. 2014 Apr 20;2014(4):CD010189. doi: 10.1002/14651858.CD010189.pub2. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2024 Nov 7;11:CD010189. doi: 10.1002/14651858.CD010189.pub3. PMID: 24748537 Free PMC article. Updated.
-
Bone marrow versus peripheral blood allogeneic haematopoietic stem cell transplantation for haematological malignancies in adults.Cochrane Database Syst Rev. 2024 Nov 7;11(11):CD010189. doi: 10.1002/14651858.CD010189.pub3. Cochrane Database Syst Rev. 2024. PMID: 39508306
-
Mycophenolate mofetil versus methotrexate for prevention of graft-versus-host disease in people receiving allogeneic hematopoietic stem cell transplantation.Cochrane Database Syst Rev. 2014 Jul 25;2014(7):CD010280. doi: 10.1002/14651858.CD010280.pub2. Cochrane Database Syst Rev. 2014. PMID: 25061777 Free PMC article.
-
Polyclonal anti-thymocyte globulins for the prophylaxis of graft-versus-host disease after allogeneic stem cell or bone marrow transplantation in adults.Cochrane Database Syst Rev. 2012 Sep 12;(9):CD009159. doi: 10.1002/14651858.CD009159.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2023 Jun 21;6:CD009159. doi: 10.1002/14651858.CD009159.pub3. PMID: 22972135 Updated.
References
-
- Satwani P, Jin Z, Duffy D, Morris E, Bhatia M, Garvin JH, George D, Bradley MB, Harrison L, Petrillo K, Schwartz J, Foley S, Hawks R, Baxter-Lowe LA, Cairo MS. Transplantation-related mortality, graft failure, and survival after reduced-toxicity conditioning and allogeneic hematopoietic stem cell transplantation in 100 consecutive pediatric recipients. Biol Blood Marrow Transplant. 2013;19:552–561. - PubMed
-
- Algeri M, Lodi M, Locatelli F. Hematopoietic Stem Cell Transplantation in Thalassemia. Hematol Oncol Clin North Am. 2023;37:413–432. - PubMed
LinkOut - more resources
Full Text Sources

