Association between novel per- and poly-fluoroalkyl substances and premature ovarian insufficiency: a case-control study
- PMID: 40740667
- PMCID: PMC12308182
- DOI: 10.1093/hropen/hoaf044
Association between novel per- and poly-fluoroalkyl substances and premature ovarian insufficiency: a case-control study
Abstract
Study question: Do novel per- and poly-fluoroalkyl substances (Novel PFAS) have associations with premature ovarian insufficiency (POI)?
Summary answer: Hexafluoropropylene oxide dimer acid (HFPO-DA), perfluorobutanoic acid (PFBA), perfluoropentanoic acid (PFPeA), and perfluoropentanesulfonic acid (PFPeS) are associated with an increased risk of POI, and the effect is worse with exposure to mixtures.
What is known already: As public health concerns following Novel PFAS exposure are rising globally, there is a need to understand the exact association between Novel PFAS and various diseases. Epidemiologic studies suggest traditional PFAS exposures adversely affect women's reproductive health, but the association between exposure to Novel PFAS and POI remains unclear.
Study design size duration: A retrospective research study, including 371 women, with (case group, n = 151) and without POI (control group, n = 220), was conducted between June 2023 and May 2024.
Participants/materials setting methods: Thirteen types of Novel PFAS and basal concentrations of FSH, LH, estradiol (E2), and anti-Müllerian hormone (AMH) in plasma samples were measured in plasma samples collected during the early follicular phase (Days 2-5) of a natural menstrual cycle. In addition, characteristics of participants were collected. Both adjusted logistic regression and Bayesian kernel machine regression (BKMR) were used to evaluate associations between Novel PFAS (alone or as a mixture) and POI. Effect modification by age was also investigated.
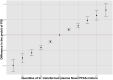
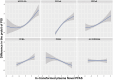
Main results and the role of chance: The concentrations of HFPO-DA, PFBA, PFPeA, and PFPeS in the case group were significantly higher than in the reference group. The adjusted logistic regression models demonstrated positive associations between plasma concentrations of HFPO-DA, PFBA, PFPeA, and PFPeS with the risk of POI [ORadj = 2.89 (95% CI: 1.84-4.53), 1.54 (95% CI: 1.17-2.02), 3.12 (95% CI: 2.20-4.43), and 2.07 (95% CI: 1.31-3.27), respectively, per 2.7-fold increase in Novel PFAS concentrations]. High concentrations of Novel PFAS showed a negative correlation with AMH and antral follicle count (AFC), but a positive correlation with FSH. After controlling for other covariates, HFPO-DA, PFBA, PFBS, PFPeA, and PFPeS were the major contributors based on the BKMR models.
Limitations reasons for caution: False positives cannot be ruled out. Therefore, experiments on PFBA, PFPeA, PFPeS, and HFPO-DA in vivo also need to be conducted in animal models.
Wider implications of the findings: Our study is the first to discover the impact of Novel PFAS on the incidence of POI, with an investigation of indicators such as AMH, FSH, and AFC. Considering increasingly severe environmental pollution, our research results provide a broader understanding of the impact of environmental endocrine disruptors on ovarian function, and suggest that women of reproductive age should reduce their exposure to Novel PFAS.
Study funding/competing interests: This work was supported by the National Key Research and Development Project of China (2022YFC2703002), National Natural Science Foundation of China (U24A20658, 82371726), Innovative Research Team of High-Level Local Universities in Shanghai (SHSMU-ZDCX20212200), Shanghai Hospital Development Center Foundation (SHDC22022303, SHDC22022201), and Key project of Medical and Industrial intersection of Shanghai Jiao Tong University (YG2023ZD27), as well as Reproductive Medicine Research Project of the Chinese Red Cross Foundation (HSZH2024GFYZQ) and Open Fund Project of Shanghai Key Laboratory of Embryogenic Diseases (shelab2023ZD02). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors declare that they have no competing interests.
Trial registration number: N/A.
Keywords: mixture models; novel per- and poly-fluoroalkyl substances (Novel PFAS); premature ovarian insufficiency; reproductive health; sex hormones.
© The Author(s) 2025. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Impact of definitive uterine artery occlusion on ovarian reserve markers in laparoscopic myomectomy: a randomized controlled trial with 2-year follow-up.Hum Reprod. 2025 Jul 1;40(7):1305-1314. doi: 10.1093/humrep/deaf070. Hum Reprod. 2025. PMID: 40420404 Free PMC article. Clinical Trial.
-
Association between endometriosis and type and age of menopause: a pooled analysis of 279 948 women from five cohort studies.Hum Reprod. 2025 Jun 1;40(6):1210-1219. doi: 10.1093/humrep/deaf068. Hum Reprod. 2025. PMID: 40304605 Free PMC article.
-
Individualised gonadotropin dose selection using markers of ovarian reserve for women undergoing in vitro fertilisation plus intracytoplasmic sperm injection (IVF/ICSI).Cochrane Database Syst Rev. 2018 Feb 1;2(2):CD012693. doi: 10.1002/14651858.CD012693.pub2. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2024 Jan 4;1:CD012693. doi: 10.1002/14651858.CD012693.pub3. PMID: 29388198 Free PMC article. Updated.
-
Randomized controlled trial to evaluate the impact of follicle priming on IVM outcomes in women with polycystic ovaries: CFA versus FSH-B.Hum Reprod. 2025 Jun 1;40(6):1127-1137. doi: 10.1093/humrep/deaf053. Hum Reprod. 2025. PMID: 40194780 Clinical Trial.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
References
-
- Ao Y, Nian M, Tang W, Zhang J, Zhang Q, Ao J. A sensitive and robust method for the simultaneous determination of thirty-three legacy and emerging per- and polyfluoroalkyl substances in human plasma and serum. Anal Bioanal Chem 2023;415:457–470. - PubMed
-
- Chaparro-Ortega A, Betancourt M, Rosas P, Vázquez-Cuevas FG, Chavira R, Bonilla E, Casas E, Ducolomb Y. Endocrine disruptor effect of perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) on porcine ovarian cell steroidogenesis. Toxicol In Vitro 2018;46:86–93. - PubMed
-
- Conley JM, Lambright CS, Evans N, McCord J, Strynar MJ, Hill D, Medlock-Kakaley E, Wilson VS, Gray LE. Hexafluoropropylene oxide-dimer acid (HFPO-DA or GenX) alters maternal and fetal glucose and lipid metabolism and produces neonatal mortality, low birthweight, and hepatomegaly in the Sprague-Dawley rat. Environ Int 2021;146:106204. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
