Liquid biopsy in breast cancer: Redefining precision medicine
- PMID: 40740670
- PMCID: PMC12308030
- DOI: 10.1016/j.jlb.2025.100312
Liquid biopsy in breast cancer: Redefining precision medicine
Abstract
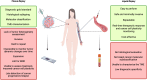
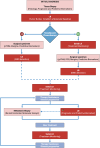
Breast cancer (BC) is the most frequent cancer and the leading cause of cancer-related death among women worldwide. It represents a heterogeneous group of diseases with distinct morphological, immunophenotypic, and molecular profiles, which significantly impact clinical behavior and therapeutic response. Moreover, under treatment pressure, tumor cells may undergo molecular changes and phenotypic plasticity, leading to resistance and therapeutic failure. Although tissue biopsy remains the gold standard for diagnosis and molecular characterization, it has several limitations, including invasiveness, sampling bias, and the inability to dynamically capture tumor evolution over time. Hence, a non-invasive and repeatable approach capable of real-time monitoring is increasingly needed. Liquid biopsy (LB), through the analysis of circulating tumor cells (CTCs) and circulating tumor DNA (ctDNA), has emerged as a powerful tool to complement tissue biopsy. It allows for longitudinal assessment of tumor burden, detection of minimal residual disease, and identification of molecular alterations relevant to targeted therapies. Despite promising results, the integration of LB into clinical practice is still limited by methodological heterogeneity, standardization gaps, and regulatory issues. Nonetheless, LB represents a key advancement toward precision oncology and may become essential in the personalized management of BC patients. In this review, we explore the current applications, benefits, and technical limitations of LB in different BC settings. We provide a comprehensive overview of the biological and clinical significance of CTCs and ctDNA, emphasizing their diagnostic, prognostic, and predictive roles. Finally, we present an updated summary of ongoing clinical trials that incorporate LB for clinical decision-making.
Keywords: Breast cancer; Circulating tumor DNA; Circulating tumor cells; Liquid biopsy.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Lafourcade A., His M., Baglietto L., Boutron-Ruault M.-C., Dossus L., Rondeau V. Factors associated with breast cancer recurrences or mortality and dynamic prediction of death using history of cancer recurrences: the French E3N cohort. BMC Cancer. 2018;18:171. doi: 10.1186/s12885-018-4076-4. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

