Assessing the iLet Bionic Pancreas Deployed in Primary Care and via Telehealth: A Randomized Clinical Trial
- PMID: 40741451
- PMCID: PMC12304562
- DOI: 10.2337/cd24-0104
Assessing the iLet Bionic Pancreas Deployed in Primary Care and via Telehealth: A Randomized Clinical Trial
Abstract
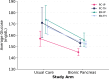
The iLet Bionic Pancreas system can improve glycemia in individuals with type 1 diabetes with its simple interface, setup, and maintenance, making it practical for use in the primary care setting and via telehealth (TH). This multisite, random-order crossover trial included 40 adults with type 1 diabetes who received care either from an endocrinology or a primary care clinic and were seen either in person or via TH. Of these 40 participants, 97% achieved an average glucose (AG) <183 mg/dL, and 64% achieved an AG <154 mg/dL. These results suggest that the system can be initiated effectively by primary care providers and via TH, potentially expanding access to this technology to a broad population, including individuals who cannot easily access subspecialty diabetes care.
© 2025 by the American Diabetes Association.
Conflict of interest statement
S.M.O. has received grants from Abbott Diabetes, Dexcom, Insulet, and The Leona M. and Harry B. Helmsley Charitable Trust outside of the submitted work and has served on advisory boards for Ascensia, Blue Circle Health, Dexcom, Eversense, Medscape, and Sequel outside of the submitted work. M.S.P. has received in-kind support and research grants from Dexcom and research grants and honoraria from Vertex Pharmaceuticals and has served on a scientific advisory board for Anagram Therapeutics, all outside of the submitted work. S.J.R. has received grants from The Leona M. and Harry B. Helmsley Charitable Trust and has equity in Beta Bionics. C.A.B. and M.A.H. have equity in Beta Bionics. E.R.D. has served on the board of directors of and has equity in Beta Bionics. T.K.O. has received grants from Abbott Diabetes, Dexcom, Insulet, and The Leona M. and Harry B. Helmsley Charitable Trust outside of the submitted work and has served on advisory boards for Ascencia, Blue Circle Health, Dexcom, Eversense, and Medscape outside of the submitted work. No other potential conflicts of interest relevant to this article were reported.
Figures
References
-
- Polonsky WH, Hood KK, Levy CJ, et al.; Omnipod 5 Research Group . How introduction of automated insulin delivery systems may influence psychosocial outcomes in adults with type 1 diabetes: findings from the first investigation with the Omnipod® 5 System. Diabetes Res Clin Pract 2022;190:109998. - PMC - PubMed
-
- Reznik Y, Bonnemaison E, Fagherazzi G, et al. The use of an automated insulin delivery system is associated with a reduction in diabetes distress and improvement in quality of life in people with type 1 diabetes. Diabetes Obes Metab 2024;26:1962–1966 - PubMed
-
- Wheeler BJ, Collyns OJ, Meier RA, et al. Improved technology satisfaction and sleep quality with Medtronic MiniMed® Advanced Hybrid Closed-Loop delivery compared to predictive low glucose suspend in people with type 1 diabetes in a randomized crossover trial. Acta Diabetol 2022;59:31–37 - PubMed
Associated data
LinkOut - more resources
Full Text Sources