FBP1 as a key regulator of focal adhesion kinase-mediated hepatic stellate cell activation: Multi-omics and experimental validation
- PMID: 40741470
- PMCID: PMC12305161
- DOI: 10.3748/wjg.v31.i28.107361
FBP1 as a key regulator of focal adhesion kinase-mediated hepatic stellate cell activation: Multi-omics and experimental validation
Abstract
Background: Inhibiting hepatic stellate cell (HSC) activation is a key therapeutic strategy in liver fibrosis (LF). During activation, aerobic glycolysis is upregulated to meet increased energy demands. Although focal adhesion kinase (FAK) has been implicated in regulating HSC glycolysis, its precise role in activation remains unclear.
Aim: To investigate the effects of FAK and fructose-1, 6-bisphosphatase 1 (FBP1) on LF through the modulation of aerobic glycolysis in HSCs.
Methods: Eighteen mice were randomly assigned to three groups: Control, carbon tetrachloride (CCl₄)-induced LF, and CCl₄ with FAK inhibitor treatment. Liver tissues were analyzed using transcriptomic and proteomic sequencing. Differential gene expression, Mfuzz clustering, and protein interaction network analyses identified key regulatory factors. Immunohistochemistry (IHC) and Western blot (WB) analysis were used to assess FAK and FBP1 expression, along with glycolysis-related enzymes. The migratory behavior of HSCs was evaluated using Transwell migration and scratch assays.
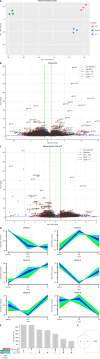
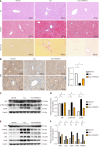
Results: Transcriptomic and proteomic analyses revealed significantly reduced FBP1 expression in CCl₄-induced fibrosis, which was restored upon FAK inhibition. Histological staining (hematoxylin and eosin, Masson's trichrome, Sirius red) confirmed reduced fibrosis following FAK inhibition. WB analysis demonstrated suppression of glycolysis-related enzymes. In LX-2 cells, FAK inhibition attenuated HSC activation and glycolysis while upregulating FBP1. Exogenous recombinant FBP1 inhibited HSC activation and glycolysis. Transwell and scratch assays showed that FBP1 significantly impaired HSC migration. In addition, WB and IHC analyses confirmed lower FBP1 expression in fibrotic liver tissues from patients compared to healthy controls.
Conclusion: FAK inhibitors and increased FBP1 expression inhibit aerobic glycolysis in HSCs, thereby improving LF. Thus, FAK and FBP1 may be potential targets for LF treatment.
Keywords: Aerobic glycolysis; Focal adhesion kinase; Fructose-1,6-bisphosphatase; Hepatic stellate cells; Liver fibrosis.
©The Author(s) 2025. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors have no conflicts of interest to declare.
Figures





References
-
- Kisseleva T, Brenner D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat Rev Gastroenterol Hepatol. 2021;18:151–166. - PubMed
-
- Ginès P, Krag A, Abraldes JG, Solà E, Fabrellas N, Kamath PS. Liver cirrhosis. Lancet. 2021;398:1359–1376. - PubMed
-
- Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14:397–411. - PubMed
-
- Ellis EL, Mann DA. Clinical evidence for the regression of liver fibrosis. J Hepatol. 2012;56:1171–1180. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

