Efficacy and Safety of Centchroman in the Treatment of Breast Fibroadenoma: A Systematic Review and Meta-Analysis
- PMID: 40741574
- PMCID: PMC12310075
- DOI: 10.7759/cureus.87046
Efficacy and Safety of Centchroman in the Treatment of Breast Fibroadenoma: A Systematic Review and Meta-Analysis
Abstract
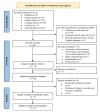
Breast fibroadenoma is a benign disease in women of reproductive age group which usually presents as painless, solitary, or multiple lumps located in the same or both breasts. Management of breast fibroadenoma ranges from watchful observation for self-regression to surgical interventions. Many studies have evaluated centchroman for its efficacy and safety in the treatment of breast fibroadenoma, but their findings were conflicting. We, therefore, aimed to conduct this systematic review and meta-analysis to resolve the conflict. The primary objective was to assess the efficacy whereas the secondary objective was to assess the safety of centchroman compared with no intervention-only watchful waiting, placebo or any active control in patients with breast fibroadenoma. The protocol for this systematic review and meta-analysis was designed a priori and registered in PROSPERO (CRD42024552160). Randomized controlled trials (RCTs), non-randomized interventional studies and prospective observational studies that evaluated efficacy and safety of centchroman in patients of breast fibroadenoma were included in the systematic review whereas only RCTs were included in the meta-analysis. Review articles, case reports and communications were excluded. A comprehensive search of databases such as Medline, Science Direct, Cochrane Library and Google Scholar and trial registries such as ClinicalTrial.gov and Clinical Trials Registry-India (CTRI) till 31/12/2024 was carried out by two independent authors. The efficacy outcome was regression of fibroadenoma measured in terms of the number of responders in each arm whereas the safety outcome was the number of patients who developed any adverse reaction. Risk ratio (RR) was used as effect measure and random effect model was used for data analysis. During the preliminary search, we found 8113 search results, of which 60 remained after removing irrelevant and duplicate results and the results available in non-English languages. We obtained full texts of 26 studies that were screened further for eligibility. Eventually, seven studies were eligible for systematic review, of which two studies were included in the meta-analysis. In the meta-analysis of two RCTs with pooled sample size of 234, the pooled estimate (RR) of efficacy outcome was 1.44 [95% CI 0.59 to 3.50; p=0.42] and heterogeneity was found to be moderate with I 2 value equal to 46%. The pooled estimate (RR) of safety outcome was 13.08 [95% CI 3.72 to 46.02; p=0.0001]. With these findings of meta-analysis, we conclude that centchroman is as efficacious as the control in causing regression of fibroadenoma and centchroman has significantly higher number of adverse effects than the control, although none of the adverse effects were serious. More RCTs with larger sample size and from diverse geographical settings are needed to generate more robust evidence and to expand generalizability over a broader population.
Keywords: breast fibroadenoma; centchroman; meta-analysis; ormeloxifene; systematic review.
Copyright © 2025, Kumar et al.
Conflict of interest statement
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures

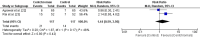
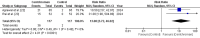
References
-
- Fibroadenoma of the breast. Houssami N, Cheung MN, Dixon JM. Med J Aust. 2001;174:185–188. - PubMed
-
- A prospective study on the role of centchroman in regression of fibroadenoma. Singla NK, Bhatia R, Verma R, Bhatia SK. https://www.clinicsinsurgery.com/open-access/a-prospective-study-on-the-... Clin Surg. 2021;6:2021–2028.
-
- A randomized, double-blind, placebo-controlled trial of ormeloxifene in breast pain and nodularity. Kumar S, Rai R, Agarwal GG, Dwivedi V, Kumar S, DAS V. https://pubmed.ncbi.nlm.nih.gov/24093978/ Natl Med J India. 2013;26:69–74. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
