Allogeneic human umbilical cord blood for acute ischemic stroke: Phase I clinical trial
- PMID: 40741608
- PMCID: PMC12306862
- DOI: 10.4103/tcmj.tcmj_249_24
Allogeneic human umbilical cord blood for acute ischemic stroke: Phase I clinical trial
Abstract
Objectives: Transplantation of human umbilical cord blood cells (hUCB) may enhance neuroprotection, and thus, the intravenous (IV) infusion of hUCB in patients with acute ischemic stroke (AIS) is being tested for its safety and efficacy.
Materials and methods: We conducted a 12-month, open-label, and single-center, phase I trial of hUCB treatment in AIS patients at the age of 45-80 years, with magnetic resonance imaging evidence of acute infarction in the internal carotid artery supplied territory and the National Institute of Health Stroke Scale (NIHSS) score between 6 and 18. Eligible participants received a single-dose IV infusion of hUCB followed by the two doses of mannitol infusion within 9 days after the onset of stroke symptoms. The primary endpoint was the incidence of adverse events (AEs) and the secondary endpoints were the changes in NIHSS, Barthel index (BI), and Berg Balance Scale (BBS) scores.
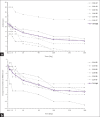
Results: Six patients (Male: Female = 3: 3) were enrolled with a mean age at 65.8 years. A total of 40 AEs occurred in six participants during this study, which included nine serious adverse events. Only transient erythema multiforme and hematuria were probably and possibly related to hUCB infusion, respectively. The mean NIHSS score was 11.5 at baseline and it significantly improved at 1, 3, 6, 9, and 12 months after treatment (mean change from baseline: -4.0, -5.3, -6.8, -7.0, and -7.3). The mean BI score was 22.5 at baseline and it significantly increased at 3 and 6 months after treatment (mean change from baseline: 26.7 and 42.5, respectively). The BBS score increased numerically but did not reach statistical significance. The changes in cytokine levels and spleen size were unremarkable.
Conclusion: The IV hUCB was safe and well tolerated in AIS patients, and the preliminary efficacy results demonstrated its therapeutic potential, supporting the conduct of a randomized, placebo controlled, phase II clinical trial in future.
Keywords: Acute ischemic stroke; Adverse events; Human umbilical cord blood; National Institute of Health Stroke Scale; Phase I clinical trial.
Copyright: © 2025 Tzu Chi Medical Journal.
Conflict of interest statement
Dr. Raymond Y. Lo, an editorial board member at Tzu Chi Medical Journal, had no role in the peer review process of or decision to publish this article. The other authors declared no conflicts of interest in writing this paper. This study was supported by StemCyte through the clinical project SCTW001, which included the provision of drugs and research funding. StemCyte had no direct involvement in the design, conduct, or reporting of this research, and the Principal Investigator (PI) maintained full independence in all aspects of the study. Chen-Yu Ko, Ying-Chieh Chen, Yu-Chin Su, Tong-Young Lee, and Chia-Tsung Wang are employees of StemCyte. The authors declare no conflicts of interest related to this work.
Figures

Similar articles
-
Safety and tolerability of intravenous liposomal GM1 in patients with Parkinson disease: A single-center open-label clinical phase I trial (NEON trial).PLoS Med. 2025 May 13;22(5):e1004472. doi: 10.1371/journal.pmed.1004472. eCollection 2025 May. PLoS Med. 2025. PMID: 40359409 Free PMC article. Clinical Trial.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Antibiotics for exacerbations of asthma.Cochrane Database Syst Rev. 2018 Jun 25;6(6):CD002741. doi: 10.1002/14651858.CD002741.pub2. Cochrane Database Syst Rev. 2018. PMID: 29938789 Free PMC article.
-
Ketamine and other glutamate receptor modulators for depression in bipolar disorder in adults.Cochrane Database Syst Rev. 2015 Sep 29;(9):CD011611. doi: 10.1002/14651858.CD011611.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2021 Oct 8;10:CD011611. doi: 10.1002/14651858.CD011611.pub3. PMID: 26415966 Updated.
-
Transcranial laser therapy for acute ischaemic stroke.Cochrane Database Syst Rev. 2025 Jul 24;7(7):CD012426. doi: 10.1002/14651858.CD012426.pub2. Cochrane Database Syst Rev. 2025. PMID: 40704566
References
-
- Newman MB, Davis CD, Kuzmin-Nichols N, Sanberg PR. Human umbilical cord blood (HUCB) cells for central nervous system repair. Neurotox Res. 2003;5:355–68. - PubMed
-
- Vendrame M, Gemma C, de Mesquita D, Collier L, Bickford PC, Sanberg CD, et al. Anti-inflammatory effects of human cord blood cells in a rat model of stroke. Stem Cells Dev. 2005;14:595–604. - PubMed
-
- Vendrame M, Gemma C, Pennypacker KR, Bickford PC, Davis Sanberg C, Sanberg PR, et al. Cord blood rescues stroke-induced changes in splenocyte phenotype and function. Exp Neurol. 2006;199:191–200. - PubMed
-
- Battistella V, de Freitas GR, da Fonseca LM, Mercante D, Gutfilen B, Goldenberg RC, et al. Safety of autologous bone marrow mononuclear cell transplantation in patients with nonacute ischemic stroke. Regen Med. 2011;6:45–52. - PubMed
LinkOut - more resources
Full Text Sources
