Efficacy and Field Safety of Ilunocitinib for the Control of Allergic Dermatitis in Client-Owned Dogs: A Multicenter, Double-Masked, Randomised, Placebo-Controlled Clinical Trial
- PMID: 40741942
- PMCID: PMC12590097
- DOI: 10.1111/vde.70009
Efficacy and Field Safety of Ilunocitinib for the Control of Allergic Dermatitis in Client-Owned Dogs: A Multicenter, Double-Masked, Randomised, Placebo-Controlled Clinical Trial
Abstract
Background: Inhibition of the Janus kinase pathway is an established treatment for allergic dermatitis.
Objective: To evaluate the efficacy and safety of ilunocitinib for control of pruritus in dogs with allergic dermatitis in a randomised, double-masked clinical trial.
Animals: Three-hundred-and-six dogs at 15 veterinary clinics.
Materials and methods: Enrolled client-owned dogs with severe pruritus and a presumptive diagnosis of allergic dermatitis were randomised to receive either ilunocitinib (n = 206; 0.6-0.8 mg/kg) or placebo (n = 100; 0 mg/kg) once daily for 28 days. Pruritus was assessed by owners using a pruritus Visual Analog Scale (PVAS). Treatment success was defined as ≥ 50% reduction from baseline PVAS on at least five of seven initial treatment days. Clinical remission from pruritus was considered achieved when PVAS < 2. Safety assessments were conducted over 112 days.
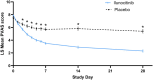
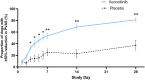
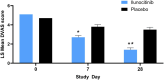
Results: On Day (D)7, 25.4% of ilunocitinib-treated dogs achieved treatment success compared to 7.7% of placebo dogs (p = 0.006). Starting on D3, the proportion of dogs with a ≥ 50% reduction from baseline PVAS was significantly higher in the ilunocitinib group (p < 0.01) and by D28, a significantly higher percentage of ilunocitinib-treated dogs (51.8%) achieved clinical remission compared to placebo dogs (12.7%; p < 0.05). Signs of dermatitis improved within 7 days. The 112-day ilunocitinib treatment was well-tolerated.
Conclusions and clinical relevance: Ilunocitinib administered once a day was well tolerated and effective at rapidly reducing pruritus, with steady and continuous improvement over time. Clinical remission of pruritus was achieved by 51.8% of ilunocitinib-treated dogs by D28, regardless of allergic aetiology.
背景: 抑制Janus激酶通路是治疗过敏性皮炎的成熟方法。 目的: 通过一项随机双盲临床试验,评估伊诺西替尼(ilunocitinib)对过敏性皮炎犬瘙痒控制的有效性和安全性。 动物: 15家兽医诊所的306只犬。 材料与方法: 入选的患有严重瘙痒且疑似诊断为过敏性皮炎的犬,随机分配接受伊诺西替尼(n = 206;0.6‐0.8 mg/kg)或安慰剂(n = 100;0 mg/kg)治疗,每日一次,持续28天。主人使用瘙痒视觉模拟量表(PVAS)评估瘙痒程度。治疗成功定义为在7个初始治疗日中至少5天,PVAS较基线减少≥50%。当 PVAS < 2 时,瘙痒症状被认为已达到临床缓解。安全性评估持续了 112 天。 结果: 在第 7 天 (D),接受伊诺西替尼治疗的犬有 25.4% 获得治疗成功,而接受安慰剂治疗的犬只有 7.7% (p = 0.006)。从第 3 天开始,伊诺西替尼组 PVAS 较基线下降 ≥ 50% 的犬比例显著高于安慰剂组 (p < 0.01);到第 28 天,接受伊诺西替尼治疗的犬 (51.8%) 获得临床缓解的比例显著高于接受安慰剂治疗的犬 (12.7%;p < 0.05)。皮炎症状在 7 天内得到改善。112 天的伊诺西替尼治疗耐受性良好。 结论及临床意义: 每日一次伊诺西替尼给药耐受性良好,可快速有效缓解瘙痒,并随着时间的推移持续稳定改善。无论过敏病因如何,接受伊诺西替尼治疗的犬在第28天时,51.8%的犬瘙痒症状达到临床缓解。.
Contexte: L'inhibition de la voie Janus kinase est un traitement établi pour la dermatite allergique.
Objectif: Évaluer l'efficacité et la sécurité d'emploi de l'ilunocitinib pour le contrôle du prurit chez les chiens atteints de dermatite allergique dans le cadre d'un essai clinique randomisé en double aveugle.
Animaux: Trois cent six chiens dans 15 cliniques vétérinaires. MATÉRIELS ET MÉTHODES: Les chiens appartenant à des clients, présentant un prurit sévère et un diagnostic présumé de dermatite allergique, ont été randomisés pour recevoir soit de l'ilunocitinib (n = 206 ; 0,6‐0,8 mg/kg), soit un placebo (n = 100 ; 0 mg/kg) une fois par jour pendant 28 jours. Le prurit a été évalué par les propriétaires à l'aide d'une échelle visuelle analogique du prurit (PVAS). Le succès du traitement a été défini comme une réduction ≥ 50 % par rapport à la PVAS de base pendant au moins cinq des sept premiers jours de traitement. La rémission clinique du prurit a été considérée comme atteinte lorsque la PVAS était < 2. Les évaluations de sécurité ont été réalisées sur 112 jours. RÉSULTATS: Au jour (J) 7, 25,4 % des chiens traités par l'ilunocitinib ont obtenu un succès thérapeutique, contre 7,7 % des chiens sous placebo (p = 0,006). À partir du jour 3, la proportion de chiens présentant une réduction ≥ 50 % par rapport au PVAS initial était significativement plus élevée dans le groupe ilunocitinib (p < 0,01) et, au jour 28, un pourcentage significativement plus élevé de chiens traités par ilunocitinib (51,8 %) ont obtenu une rémission clinique par rapport aux chiens sous placebo (12,7 % ; p < 0,05). Les signes de dermatite se sont améliorés en 7 jours. Le traitement par ilunocitinib pendant 112 jours a été bien toléré.
Conclusions et pertinence clinique: L'ilunocitinib administré une fois par jour a été bien toléré et s'est révélé efficace pour réduire rapidement le prurit, avec une amélioration constante et continue au fil du temps. Une rémission clinique du prurit a été obtenue chez 51,8 % des chiens traités par l'ilunocitinib au jour 28, quelle que soit l'étiologie allergique.
Hintergrund: Die Inhibition des Januskinase Zyklus ist eine etablierte Behandlungsmethode der allergischen Dermatitis.
Ziele: Die Evaluierung der Wirksamkeit und der Sicherheit von Ilunocitinib zur Juckreizkontrolle bei Hunden mit allergischer Dermatitis in einer randomisierten, doppel‐blinden klinischen Studie.
Tiere: Dreihundertsechs Hunde aus 15 veterinärmedizinischen Kliniken.
Materialien und methoden: Die in die Studie aufgenommenen Privathunde mit hochgradigem Juckreiz und der vermeintlichen Diagnose einer allergischen Dermatitis wurden zufällig in Gruppen eingeteilt, um entweder Ilunocitinib (n = 206; 0,6‐0,8 mg/kg) oder Placebo (n = 100; 0 mg/kg) einmal täglich für 28 Tage zu erhalten. Der Pruritus wurde durch die BesitzerInnen mittels einer Pruritus Visual Analog Scale (PVAS) erfasst. Der Behandlungserfolg wurde mit einer ≥50%igen Reduzierung vom Ausgangspunkt der PVAS an mindestens fünf von sieben anfänglichen Behandlungstagen definiert. Eine klinische Remission des Pruritus wurde bei PVAS < 2 erreicht. Sicherheitsbewertungen wurden über eine Dauer von 112 Tagen durchgeführt.
Ergebnisse: Am Tag (D)7 erzielten 25,4% der mit Ilunocitinib‐behandelten Hunde im Vergleich zu 7,7% der mit Placebo behandelten Hunden einen Behandlungserfolg (p = 0,006). Ab D3 war der Anteil der Hunde mit einer ≥50%igen Reduzierung vom Ausgangswert des PVAS in der Ilunocitinib Gruppe signifikant höher (p < 0,01) und mit D28 erzielte ein signifikant höherer Prozentsatz von Ilunocitinib‐behandelten Hunden (51,8%) eine klinische Remission im Vergleich zu den Placebo Hunden (12,7%; p < 0,05). Die Zeichen einer Dermatitis verbesserten sich innerhalb von 7 Tagen. Die 112‐Tage andauernde Behandlung mit Ilunocitinib wurde gut toleriert.
Schlussfolgerungen und klinische bedeutung: Ilunocitinib wurde bei einer einmal täglichen Verabreichung gut toleriert und reduzierte den Juckreiz wirksam und rasch, wobei mit der Zeit eine beständige und kontinuierliche Verbesserung erfolgte. Eine klinische Remission des Pruritus wurde unabhängig von der allergischen Ätiologie von 51,8% der mit Ilunocitinib‐behandelten Hunde am D28 erzielt.
背景: ヤヌスキナーゼ経路の阻害はアレルギー性皮膚炎の治療法として確立されている。 目的: 本研究の目的は、イルノシチニブのアレルギー性皮膚炎犬の掻痒抑制に対する有効性および安全性を無作為化二重マスク臨床試験で評価することであった。 対象動物: 15の動物病院における36頭の犬。 材料と方法: 重度の掻痒を有し、アレルギー性皮膚炎と推定される犬を登録し、イルノシチニブ(n = 206; 0.6‐0.8 mg/kg)またはプラセボ(n = 100; 0 mg/kg)を1日1回28日間投与する群に無作為に割り付けた。掻痒は、オーナーが装用のVisual Analog Scale(PVAS)を用いて評価した。治療成功の定義は、初回治療7日間のうち少なくとも5日間においてPVASがベースラインから50%以上減少したこととした。安全性評価は112日間にわたって実施された。 結果: Day(D)7において、イルノシチニブ投与犬の25.4%が治療成功を達成したのに対し、プラセボ投与犬では7.7%であった(p = 0.006)。D3以降、ベースラインからPVASが50%以上減少した犬の割合はイルノシチニブ群で有意に高く(p<0.01)、D28までに臨床的寛解を達成した犬の割合は、プラセボ群(12.7%;p<0.05)に比べイルノシチニブ群(51.8%)で有意に高かった。皮膚炎の徴候は7日以内に改善した。112日間のイルノシチニブ治療は良好な忍容性を示した。 結論と臨床的意義: イルノシチニブの1日1回投与は忍容性が高く、掻痒を速やかに軽減させる効果があり、時間の経過とともに着実に継続的に改善した。アレルギー性病因にかかわらず、イルノシチニブ投与犬の51.8%がD28までに臨床的寛解を達成した。.
Contexto: A inibição da via da Janus quinase é um tratamento estabelecido para dermatite alérgica.
Objetivo: Avaliar a eficácia e a segurança do ilunocitinib no controle do prurido em cães com dermatite alérgica em um ensaio clínico randomizado e duplo‐cego.
Animais: Trezentos e seis cães em 15 clínicas veterinárias. MATERIAIS E MÉTODOS: Cães de proprietários, com prurido intenso e diagnóstico presuntivo de dermatite alérgica, foram randomizados para receber ilunocitinib (n = 206; 0,6‐0,8 mg/kg) ou placebo (n = 100; 0 mg/kg) uma vez ao dia durante 28 dias. O prurido foi avaliado pelos tutores por meio da Escala Visual Analógica de Prurido (PVAS). O sucesso do tratamento foi definido como redução ≥ 50% em relação à PVAS basal em pelo menos cinco dos sete dias iniciais de tratamento. A remissão clínica do prurido foi considerada alcançada quando a PVAS foi < 2. As avaliações de segurança foram realizadas ao longo de 112 dias.
Resultados: No Dia (D) 7, 25,4% dos cães tratados com ilunocitinib obtiveram sucesso no tratamento, em comparação com 7,7% dos cães que receberam placebo (p = 0,006). A partir do D3, a proporção de cães com redução ≥ 50% da PVAS basal foi significativamente maior no grupo ilunocitinib (p < 0,01) e, no D28, uma porcentagem significativamente maior de cães tratados com ilunocitinib (51,8%) alcançou remissão clínica em comparação com os cães que receberam placebo (12,7%; p < 0,05). Os sinais de dermatite melhoraram em 7 dias. O tratamento de 112 dias com ilunocitinib foi bem tolerado. CONCLUSÕES E RELEVÂNCIA CLÍNICA: O ilunocitinib administrado uma vez ao dia foi bem tolerado e eficaz na rápida redução do prurido, com melhora constante e contínua ao longo do tempo. A remissão clínica do prurido foi alcançada por 51,8% dos cães tratados com ilunocitinib até o dia 28, independentemente da etiologia alérgica.
INTRODUCCIÓN: La inhibición de la vía de la quinasa Janus es un tratamiento establecido para la dermatitis alérgica.
Objetivo: Evaluar la eficacia y seguridad de ilunocitinib para el control del prurito en perros con dermatitis alérgica en un ensayo clínico al azar y doble ciego.
Animales: Trescientos seis perros en 15 clínicas veterinarias. MATERIALES Y MÉTODOS: Se incluyeron perros de propietarios particulares con prurito severo y diagnóstico presuntivo de dermatitis alérgica, que fueron distribuidos al azar para recibir ilunocitinib (n = 206; 0,6‐0,8 mg/kg) o placebo (n = 100; 0 mg/kg) una vez al día durante 28 días. El prurito fue evaluado por los propietarios mediante la Escala Visual Análoga del Prurito (PVAS). El éxito del tratamiento se definió como una reducción ≥50% con respecto a la PVAS basal en al menos cinco de los siete días iniciales de tratamiento. Se consideró que se había alcanzado la remisión clínica del prurito cuando el PVAS era < 2. Se realizaron evaluaciones de efectos adversos durante 112 días.
Resultados: El día 7, el 25,4 % de los perros tratados con ilunocitinib alcanzaron el éxito del tratamiento, en comparación con el 7,7 % de los perros que recibieron placebo (p = 0,006). A partir del día 3, la proporción de perros con una reducción ≥50 % del PVAS basal fue significativamente mayor en el grupo de ilunocitinib (p < 0,01) y, para el día 28, un porcentaje significativamente mayor de perros tratados con ilunocitinib (51,8 %) alcanzó la remisión clínica en comparación con los perros que recibieron placebo (12,7 %; p < 0,05). Los signos de dermatitis mejoraron en 7 días. El tratamiento con ilunocitinib durante 112 días fue bien tolerado. CONCLUSIONES Y RELEVANCIA CLÍNICA: La administración diaria de ilunocitinib fue bien tolerada y eficaz para reducir rápidamente el prurito, con una mejoría constante y progresiva. El 51,8 % de los perros tratados con ilunocitinib alcanzó la remisión clínica del prurito al día 28, independientemente de la etiología alérgica.
Keywords: JAK inhibitor; allergic dermatitis; clinical remission; ilunocitinib; pruritus; skin lesions.
© 2025 Elanco Animal Health. Veterinary Dermatology published by John Wiley & Sons Ltd on behalf of ESVD and ACVD.
Conflict of interest statement
All authors are current or former employees of Elanco Animal Health.
Figures
References
-
- Bensignor E., Marignac G., Crosaz O., and Cavana P., “Pruritus in Dogs,” Veterinary Dermatology 24 (2013): 292. - PubMed
-
- Favrot C., Steffan J., Seewald W., and Picco F., “A Prospective Study on the Clinical Features of Chronic Canine Atopic Dermatitis and Its Diagnosis,” Veterinary Dermatology 21 (2010): 23–31. - PubMed
-
- Marsella R., “Advances in Our Understanding of Canine Atopic Dermatitis,” Veterinary Dermatology 32 (2021): 547.e151. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

