Clinical pharmacokinetics of metronidazole: a systematic review and meta-analysis
- PMID: 40741956
- PMCID: PMC12406673
- DOI: 10.1128/aac.01904-24
Clinical pharmacokinetics of metronidazole: a systematic review and meta-analysis
Abstract
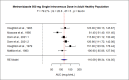
Metronidazole (MTZ) is used in various clinical settings; however, its pharmacokinetics may vary across patient populations due to physiological and pathological differences. Understanding these variations is important for personalized dosing and optimization of therapeutic outcomes. This study aimed to systematically review clinical pharmacokinetic studies of MTZ and perform a meta-analysis of the area under the concentration-time curve (AUC). AUC was selected for meta-analysis as it provides a direct and comprehensive measure of total drug exposure over time, facilitating standardized comparisons across populations. Four databases, including PubMed, ScienceDirect, Cochrane Library, and Google Scholar, were screened for pharmacokinetic studies on MTZ using systematic search strategies until July 2024. Out of 1,882 articles identified in the literature search, only 67 studies that fulfilled eligibility criteria were included in this systematic review. A meta-analysis of AUC was performed using random-effects models, with heterogeneity assessed by I² statistic. Effect sizes (pooled AUC) were compared across populations and visually presented with their corresponding 95% confidence intervals. Meta-analysis revealed significant differences in AUC across populations, with substantial heterogeneity among studies. This study provides a comprehensive evaluation of the MTZ pharmacokinetic profile across diverse patient populations. The findings emphasize the importance of tailored dosing strategies and support evidence-based clinical decision-making for optimizing the safety and efficacy of MTZ.
Keywords: AUC; Cmax; clearance; metronidazole; pharmacokinetics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures













References
-
- Fonnes S, Weisser JJ, Holzknecht BJ, Arpi M, Rosenberg J. 2020. The plasma pharmacokinetics of fosfomycin and metronidazole after intraperitoneal administration in patients undergoing appendectomy for uncomplicated appendicitis. Fundam Clin Pharmacol 34:504–512. doi: 10.1111/fcp.12535 - DOI - PubMed
-
- Shinn DLS. 1962. Metronidazole in acute ulcerative gingivitis. The Lancet 279:1191. doi: 10.1016/S0140-6736(62)92243-2 - DOI
-
- Weir CB, Le JK. 2019. Metronidazole. - PubMed
-
- Drug.com . 2023. Metronidazole. Available from: https://www.drugs.com/metronidazole.html. Retrieved Aug 25
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

