Hypothalamic gliosis as a potential mediator of improved glucose tolerance induced by time-restricted feeding in obese mice
- PMID: 40741982
- PMCID: PMC12377117
- DOI: 10.1152/ajpcell.00357.2025
Hypothalamic gliosis as a potential mediator of improved glucose tolerance induced by time-restricted feeding in obese mice
Abstract
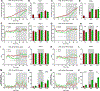
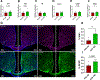
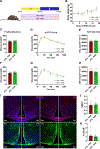
Time-restricted feeding (TRF) has been shown to improve glycemic control, reduce liver fat, and decrease cardiovascular risk in humans and diet-induced obese (DIO) mice. However, the mechanisms associated with this improvement are not completely understood. High-fat diet (HFD)-associated hypothalamic inflammation and glial activation promote obesity and metabolic dysfunction, raising the possibility that TRF mitigates these factors. Specifically, TRF increases circulating levels of β-hydroxybutyrate (BHB), a ketone body that crosses the blood-brain barrier and has anti-inflammatory properties. Here, we evaluated whether the beneficial effects of TRF are associated with changes in hypothalamic inflammation and gliosis. Furthermore, we assessed the ability of peripheral or central administration of BHB to mimic the metabolic phenotype of TRF. Consistent with prior studies in HFD-fed mice, weight loss induced by TRF was modest, due to a transient decrease in food intake offset by a persistent reduction in energy expenditure. Despite the limited effect on body weight and adiposity, TRF markedly improved glucose tolerance in DIO mice, restoring glucose homeostasis to the level of chow-fed controls. Unexpectedly, TRF increased hypothalamic markers of gliosis in DIO mice. Finally, although TRF induced the predicted rise in circulating BHB levels, chronic systemic or ICV administration of BHB had no effect on glucose tolerance and hypothalamic gliosis. Together, these data suggest that increased hypothalamic gliosis may contribute to the improvement of glucose tolerance induced by TRF in DIO mice.NEW & NOTEWORTHY This study shows that time-restricted feeding (TRF) improves glucose tolerance in obese mice independently of weight loss. Surprisingly, this benefit is linked to increased hypothalamic gliosis, challenging the view that gliosis is solely detrimental in obesity. Although TRF elevates circulating β-hydroxybutyrate (BHB), peripheral and central BHB delivery fails to mimic TRF's glycemic benefits or affect hypothalamic gliosis. These findings suggest gliosis may play a previously unrecognized role in mediating TRF's metabolic benefits.
Keywords: hypothalamic inflammation; obesity; time-restricted feeding; β-hydroxybutyrate.
Conflict of interest statement
Declaration of competing interest
The authors state that they have no potential conflicts of interest, whether financial or otherwise.
Figures




References
-
- Hutchison AT, Regmi P, Manoogian ENC, Fleischer JG, Wittert GA, Panda S, and Heilbronn LK. Time-Restricted Feeding Improves Glucose Tolerance in Men at Risk for Type 2 Diabetes: A Randomized Crossover Trial. Obesity (Silver Spring) 27: 724–732, 2019. - PubMed
MeSH terms
Substances
Grants and funding
- R01 DK119754/DK/NIDDK NIH HHS/United States
- R21DK127296/HHS | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
- 21191535/Agencia Nacional de Investigación y Desarrollo (ANID)
- R01DK119754/HHS | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
- P30 DK035816/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

