The diverse phenotypic and mutational landscape induced by fluoroquinolone treatment
- PMID: 40742148
- PMCID: PMC12363166
- DOI: 10.1128/msystems.00713-25
The diverse phenotypic and mutational landscape induced by fluoroquinolone treatment
Abstract
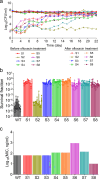
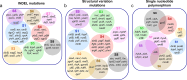
Antibiotic resistance remains a major public health challenge, yet the broader effects of antibiotic treatment on bacterial tolerance, resistance, and fitness are not fully understood. In this study, we investigated how Escherichia coli adapts to fluoroquinolone stress using adaptive laboratory evolution. Cell populations were subjected to repeated cycles of high-dose ofloxacin exposure followed by drug-free recovery, a dynamic model that imposes alternating selective pressure and inter-strain competition, reflecting real-world clinical or environmental conditions. Our results demonstrate that tolerance and resistance can evolve independently, even under identical conditions, leading to diverse phenotypic outcomes. Notably, we observed the emergence of mutants with high ofloxacin tolerance but reduced minimum inhibitory concentrations, an outcome that challenges conventional understanding. Fitness traits, including lag phase duration, doubling time, competition score, redox activity, and ATP levels, were variably affected across evolved strains, with no consistent correlation between fitness and tolerance or resistance. Whole-genome sequencing revealed both known and novel mutations, with limited convergence across populations. For example, while mutations in the icd gene were commonly observed, many other mutations, including in cyoE, lgoT, yghC, rnd, dld, and uidB, were unique to individual lineages. The lack of convergence across evolved populations may reflect the influence of competitive dynamics during recovery phases, where differing growth advantages shape selection in parallel with antibiotic pressure. These findings underscore the complexity of microbial adaptation and highlight how fluctuating environments and population-level interactions can drive non-uniform evolutionary outcomes.IMPORTANCEAntibiotic resistance poses a critical global health threat, with antibiotic-tolerant cells further complicating treatment by promoting infection relapse and enabling resistance mutations. Though tolerant cells can evolve into resistant strains, their phenotypic and genotypic characteristics are still poorly understood. In this study, we used adaptive laboratory evolution to generate several distinct ofloxacin-resistant mutants and examined their fitness (e.g., lag phase), metabolic traits (e.g., ATP levels), and genetic adaptations through whole-genome sequencing. We uncovered novel findings, including highly tolerant mutants exhibiting unexpectedly low minimum inhibitory concentrations and others with shorter lag phases, challenging conventional patterns in bacterial resistance evolution. Our findings provide critical insights into the diverse pathways and mechanisms underpinning bacterial adaptation, underscoring the complexity of resistance evolution.
Keywords: adaptive laboratory evolution; antibiotic resistance; antibiotic tolerance; bacterial fitness factors; fluoroquinolone; mutagenesis; resistant mutants.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Interventions to improve safe and effective medicines use by consumers: an overview of systematic reviews.Cochrane Database Syst Rev. 2014 Apr 29;2014(4):CD007768. doi: 10.1002/14651858.CD007768.pub3. Cochrane Database Syst Rev. 2014. PMID: 24777444 Free PMC article.
-
Falls prevention interventions for community-dwelling older adults: systematic review and meta-analysis of benefits, harms, and patient values and preferences.Syst Rev. 2024 Nov 26;13(1):289. doi: 10.1186/s13643-024-02681-3. Syst Rev. 2024. PMID: 39593159 Free PMC article.
-
Diverse impacts of different rpoB mutations on the anti-tuberculosis efficacy of capreomycin.EBioMedicine. 2025 Jul;117:105776. doi: 10.1016/j.ebiom.2025.105776. Epub 2025 May 30. EBioMedicine. 2025. PMID: 40449326 Free PMC article.
-
Recurrent mutations drive the rapid evolution of pesticide resistance in the two-spotted spider mite Tetranychus urticae.Elife. 2025 Aug 11;14:RP106288. doi: 10.7554/eLife.106288. Elife. 2025. PMID: 40788297 Free PMC article.
References
-
- Coque TM, Graham DW, Pruden A. 2023. Bracing for superbugs: strengthening environmental action in the One Health response to antimicrobial resistance. In So ET, Grooters SV (ed), United Nations Environment Programme. Newcastle University.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

