Effects of parental care on skin microbial community composition in poison frogs
- PMID: 40742751
- PMCID: PMC12313234
- DOI: 10.7554/eLife.103331
Effects of parental care on skin microbial community composition in poison frogs
Abstract
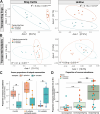
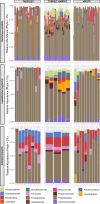
Parent-offspring interactions constitute the first contact of many newborns with their environment, priming community assembly of microbes through priority effects and shaping host health and disease. Microbe acquisition during parental care is well studied in humans and agriculturally relevant species but remains poorly understood in other vertebrate groups, such as amphibians. Here, we investigate vertical transmission of skin microbiota in poison frogs (Dendrobatidae), where fathers transport tadpoles piggyback-style from terrestrial clutches to aquatic nurseries. We found that substantial bacterial colonization of embryos begins after hatching, suggesting that the vitelline envelope acts as a microbial barrier. A cross-foster experiment demonstrated that poison frogs performing tadpole transport serve as a source of skin microbes for tadpoles on their back. To study how transport impacts skin communities of tadpoles in an ecologically relevant setting, we sampled sympatric species that do or do not exhibit tadpole transport in their natural habitat. We did not find a higher degree of similarity between microbial communities of tadpoles and adults in species that transport their offspring compared to those that do not. Similarly, communities of tadpoles were no more similar to their caregiver than to unrelated adults, indicating that most caregiver-associated microbes do not remain in tadpole communities long-term. Nonetheless, some taxa persisted on tadpoles over development. This study is the first to demonstrate that offspring transport facilitates transmission of parental skin microbes in anurans.
Keywords: ecology; infectious disease; microbiology; microbiome; poison frogs; tadpole transport; vertical transmission.
Plain language summary
The first microbes that animals encounter after birth can impact their health and development for life. In many species, these microbes are passed from parent to offspring during close contact or while caring for the young – a process known as vertical transmission. While this has been well studied in mammals, we know much less about how, or even if, microbes are passed from parent to offspring in amphibians. Amphibians rely on healthy skin to breathe and regulate water and salt levels, and skin-dwelling microbes also help protect them from infections. Many tropical frogs also display striking forms of parental care. For example, many poison frog parents carry their newly hatched tadpoles from land to small pools of water on their backs. This journey can take hours or even days, creating a perfect opportunity for microbes to transfer from parent to offspring through skin-to-skin contact. Despite this, it remains unclear how parental care in frogs influences the composition of microbes in their offspring. To find out more, Fischer et al. explored when and how poison frog tadpoles acquire their skin microbes. First, the researchers examined whether frog embryos are exposed to microbes while still developing inside the egg. Then they tested whether physical contact during transport – when the parent carries the tadpole – actually passes microbes to the tadpole, and whether those microbes remain on the tadpole’s skin as it grows. To answer these questions, Fischer et al. combined lab experiments with field research. Using DNA sequencing to study the bacteria living on tadpole skin, Fischer et al. found that most microbes begin colonizing only after the tadpoles hatch from their egg membranes, just before they are carried to water by a parent. In laboratory experiments, tadpoles picked up microbes through direct contact with the skin of the parent carrying them. In wild frogs, it was found that while microbes do transfer, only a few persisted on the tadpoles’ skin over time. These findings are important for understanding how microbial communities are formed and how parental care shapes this process. For frogs, skin microbes provide a crucial defense against deadly diseases such as chytrid fungus. By understanding how and when amphibians acquire beneficial microbes, we can help protect the habitats and behaviors that support this process and better conserve vulnerable species.
© 2025, Fischer et al.
Conflict of interest statement
MF, KX, EC, MD, GR, AR, SC, DR No competing interests declared, LO Reviewing editor, eLife
Figures






Update of
-
Effects of parental care on skin microbial community composition in poison frogs.bioRxiv [Preprint]. 2025 May 21:2024.09.11.612488. doi: 10.1101/2024.09.11.612488. bioRxiv. 2025. Update in: Elife. 2025 Jul 31;14:RP103331. doi: 10.7554/eLife.103331. PMID: 39314287 Free PMC article. Updated. Preprint.
Similar articles
-
Effects of parental care on skin microbial community composition in poison frogs.bioRxiv [Preprint]. 2025 May 21:2024.09.11.612488. doi: 10.1101/2024.09.11.612488. bioRxiv. 2025. Update in: Elife. 2025 Jul 31;14:RP103331. doi: 10.7554/eLife.103331. PMID: 39314287 Free PMC article. Updated. Preprint.
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Adapting Safety Plans for Autistic Adults with Involvement from the Autism Community.Autism Adulthood. 2025 May 28;7(3):293-302. doi: 10.1089/aut.2023.0124. eCollection 2025 Jun. Autism Adulthood. 2025. PMID: 40539213
-
How lived experiences of illness trajectories, burdens of treatment, and social inequalities shape service user and caregiver participation in health and social care: a theory-informed qualitative evidence synthesis.Health Soc Care Deliv Res. 2025 Jun;13(24):1-120. doi: 10.3310/HGTQ8159. Health Soc Care Deliv Res. 2025. PMID: 40548558
References
-
- Abarenkov K, Zirk A, Piirmann T, Pöhönen R, Ivanov F, Nilsson RH, Kõljalg U. 2023. UNITE General FASTA Release for Eukaryotes. UNITE Community. - DOI
-
- Adobe Inc Adobe illustrator. 28.3Adobe. 2024 https://www.adobe.com/products/illustrator.html
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

