A multi-biomarker approach to risk stratification and detection of early cardiac disease in systemic sclerosis
- PMID: 40743121
- PMCID: PMC12312888
- DOI: 10.1371/journal.pone.0328734
A multi-biomarker approach to risk stratification and detection of early cardiac disease in systemic sclerosis
Abstract
Objective: We sought to investigate the relationship between serum biomarkers of cardiac dysfunction, longitudinal strain on echocardiography, and all-cause mortality in patients with systemic sclerosis.
Methods: This was an observational study using a biorepository of serum samples of patients with systemic sclerosis who underwent echocardiography. We investigated 3 biomarkers: periostin, galectin-3, and N-terminal prohormone brain natriuretic peptide and applied a K-means clustering resulting in 3 patient clusters. We subsequently measured left ventricular and right ventricular free wall longitudinal strain in each cluster. We then determined the association between each cluster and time to all-cause mortality compared to N-terminal prohormone brain natriuretic peptide, alone.
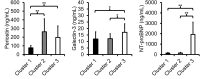
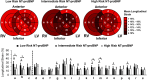
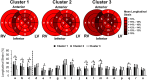
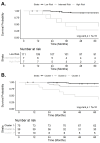
Results: The 125 patients with systemic sclerosis included in the study were divided into 3 clusters based on biomarker levels (Cluster 1: N = 75; Cluster 2: N = 39; Cluster 3: N = 11). Compared to Cluster 1, Cluster 2 had only elevated periostin levels whereas Cluster 3 had elevated levels of all 3 serum biomarkers and was characterized by reduced left ventricular and right ventricular free wall longitudinal strain, regionally and globally. When adjusted for age, sex, systemic sclerosis disease duration, and forced vital capacity, patients in Cluster 3 had a HR of 14.42 (95% CI: 4.82, 43.18) for all-cause mortality compared to those in Cluster 1.
Conclusion: In conclusion, combining N-terminal prohormone brain natriuretic peptide, periostin, and galectin-3 as serum biomarkers enhances risk stratification and sensitivity in detection of cardiac disease in patients with systemic sclerosis. However, before implementation in routine care, further prospective studies must refine biomarker sensitivity, specificity, and accuracy together with optimizing detection strategies and establishing clinical protocols for integration.
Copyright: © 2025 Lui et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
J. K. L. has received research support from United Therapeutics. E. S. K. has received research support from Novartis, FORMA Therapeutics/Novo Nordisk, and United Therapeutics. She received royalties for 3 topic cards in UpToDate. She is a consultant/advisory board member for Pfizer, Novo Nordisk, and CSL Behring for sickle cell disease related clinical trials (no conflict with the present work).
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

