Comprehensive analysis of Toxoplasma gondii migration routes and tissue dissemination in the host
- PMID: 40743275
- PMCID: PMC12312914
- DOI: 10.1371/journal.pntd.0013369
Comprehensive analysis of Toxoplasma gondii migration routes and tissue dissemination in the host
Abstract
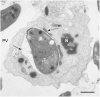
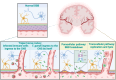
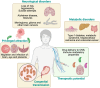
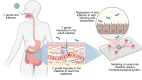
Toxoplasma gondii is a highly adaptable intracellular parasite capable of infecting a wide range of warm-blooded animals, including humans. Following the ingestion of cysts and oocysts, the parasites rapidly emerge and transmigrate through the bloodstream, initiating a complex infection process. Despite reports on the parasite's dissemination, the mechanisms behind its migration remain unclear. Recent advances using innovative 3D models and various host systems are beginning to shed light on the migratory routes and strategies employed by T. gondii. This review compiles current knowledge on the migration and dissemination of T. gondii, from its initial interactions in the gut to its invasion of immune-privileged organs. This review provides a comprehensive understanding of how T. gondii establishes its infection crossing the most impermeable biological barriers within the host.
Copyright: © 2025 Ramírez-Flores, Mondragón-Flores. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Toxoplasma gondii Dissemination in the Brain Is Facilitated by Infiltrating Peripheral Immune Cells.mBio. 2022 Dec 20;13(6):e0283822. doi: 10.1128/mbio.02838-22. Epub 2022 Nov 29. mBio. 2022. PMID: 36445695 Free PMC article.
-
A cathepsin C-like protease mediates the post-translation modification of Toxoplasma gondii secretory proteins for optimal invasion and egress.mBio. 2023 Aug 31;14(4):e0017423. doi: 10.1128/mbio.00174-23. Epub 2023 Jun 16. mBio. 2023. PMID: 37326431 Free PMC article.
-
Injection with Toxoplasma gondii protein affects neuron health and survival.Elife. 2021 Jun 9;10:e67681. doi: 10.7554/eLife.67681. Elife. 2021. PMID: 34106047 Free PMC article.
-
Toxoplasma gondii Infection in the Male Reproductive System: A Systematic Review.Acta Parasitol. 2025 Jan 24;70(1):29. doi: 10.1007/s11686-024-00978-w. Acta Parasitol. 2025. PMID: 39853507
-
A systematic review and meta-analysis of the seroprevalence of Toxoplasma gondii in cats in mainland China.Parasit Vectors. 2017 Jan 13;10(1):27. doi: 10.1186/s13071-017-1970-6. Parasit Vectors. 2017. PMID: 28086987 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

