Unknown Immunoregulatory Effects of FcRn Inhibition by Efgartigimod in Myasthenia Gravis: A New Mechanism of Action Beyond IgG Reduction
- PMID: 40743488
- PMCID: PMC12316462
- DOI: 10.1212/NXI.0000000000200455
Unknown Immunoregulatory Effects of FcRn Inhibition by Efgartigimod in Myasthenia Gravis: A New Mechanism of Action Beyond IgG Reduction
Abstract
Background and objectives: Efgartigimod (EFG), a biological drug targeting the IgG recycling neonatal Fc receptor (FcRn), leads to clinical improvements in patients affected by myasthenia gravis (MG), a prototypic autoantibody (Ab)-mediated autoimmune disease affecting neuromuscular junction. Because FcRn is a multifunctional protein expressed in different immune system cells, including B cells, we investigated whether FcRn blockade by EFG may have further immunologic effects, other than IgG reduction, in patients with MG.
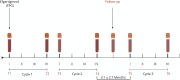
Methods: Anti-acetylcholine receptor Ab-positive (AChR-MG) patients were treated with EFG according to the GENERATIVE protocol. Clinical evaluation, IgG and autoAb quantification, and circulating T-cell and B-cell subpopulation analyses by flow cytometry were performed at different time points. The expression of regulatory plasma cell-related candidate genes (CD38, lymphocyte-activation gene 3 [LAG3], IL-12a, Ebi3) was assessed by real-time PCR in peripheral blood mononuclear cells (PBMCs) from patients on treatment and in PBMCs either untreated or in vitro treated with an EFG-mimicking anti-FcRn monoclonal Ab (mAb) or with EFG (Vyvgart).
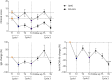
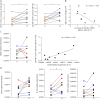
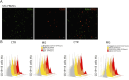
Results: A significantly increased percentage of CD19+/CD27+ memory B cells and CD27+/CD138+ plasma cells was observed at the end of EFG treatment cycle 1 and cycle 2 in patients with AChR-MG. Plasma cell increase, maintained up to cycle 3, significantly correlated with Quantitative Myasthenia Gravis score improvement. Moreover, PBMCs from EFG-treated patients showed overexpression of CD38, LAG3, and IL-12a genes, suggesting EFG's ability to induce non-pathogenic regulatory plasma cells. This ability was confirmed in vitro, because anti-FcRn mAb-treated and EFG-treated PBMCs displayed an up-regulation of CD38 and LAG3 compared with untreated cells.
Discussion: Our findings indicate an unknown immunoregulatory action of EFG in patients with AChR-MG, by unraveling a drug effect on B-cell differentiation, and suggest the induction of regulatory plasma cells as a further mechanism, beyond IgG reduction, associated with clinical improvement. A deep understanding of the immunologic effects of EFG can help to optimize its usage over time in individual patients and disclose biomarkers suitable for monitoring the long-term patient-specific response.
Conflict of interest statement
R. Frangiamore has received funding for consulting and speaking from Alexion Pharmaceuticals, UCB, and Argenx. S. Bonanno has received funding for travel, meeting attendance, and advisory board participation from Alexion; Sanofi; Roche; and Biogen. L. Maggi has received funding for travel, meeting attendance, and advisory board participation from Alexion; Sanofi Genzyme; Amicus Therapeutics; Janssen; Argenx; Biogen; Lupin; Roche; patient-centered biopharmaceutical company (PTC) therapeutics; and UCB. F. Vanoli has received funding for travel, meeting attendance, and advisory board participation from Alexion; UCB Pharma; Argenx; and Sanofi. R. Mantegazza has received compensation for participating in advisory boards in relation to MG clinical trial designs, congress participations, and research support from Alexion Pharmaceuticals; ARGENX Pharma; UCB; and PIEM. C. Antozzi has received funding for travel, meeting attendance, and advisory board participation from Alexion; Momenta; Sanofi; Janssen; Argenx; and UCB. P. Cavalcante has received compensation for participating in advisory boards and speaking at scientific meetings from Alexion Pharmaceuticals. The authors are also part of the European Reference Network for rare neuromuscular diseases (ERN-NMD). Go to
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
