Coupling IL-2 with IL-10 to mitigate toxicity and enhance antitumor immunity
- PMID: 40744022
- PMCID: PMC12432382
- DOI: 10.1016/j.xcrm.2025.102257
Coupling IL-2 with IL-10 to mitigate toxicity and enhance antitumor immunity
Abstract
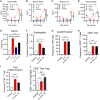
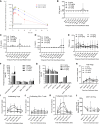
Wild-type interleukin (IL)-2 induces anti-tumor immunity and toxicity, predominated by vascular leak syndrome (VLS) leading to edema, hypotension, organ toxicity, and regulatory T cell (Treg) expansion. Efforts to uncouple IL-2 toxicity from its potency have failed in the clinic. We hypothesize that IL-2 toxicity is driven by cytokine release syndrome (CRS) followed by VLS and that coupling IL-2 with IL-10 will ameliorate toxicity. Our data, generated using human primary cells, mouse models, and non-human primates, suggest that coupling of these cytokines prevents toxicity while retaining cytotoxic T cell activation and limiting Treg expansion. In syngeneic murine tumor models, DK210 epidermal growth factor receptor (EGFR), an IL-2/IL-10 fusion molecule targeted to EGFR via an anti-EGFR single-chain variable fragment (scFV), potently activates T cells and natural killer (NK) cells and elicits interferon (IFN)γ-dependent anti-tumor function without peripheral inflammatory toxicity or Treg accumulation. Therefore, combining IL-2 with IL-10 uncouples toxicity from immune activation, leading to a balanced and pleiotropic anti-tumor immune response.
Keywords: EGFR; IFNγ; IL-10; IL-2; T cell exhaustion; cytokine; cytokine release syndrome; immunotherapy; targeted therapy; vascular leak syndrome.
Copyright © 2025 Deka Biosciences. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests J.B.M., C.H.Y., and P.A.K. are current employees and shareholders at Deka Biosciences (“Deka”). Every author on this submission has been granted employee stock options as a part of their employment with Deka. J.B.M. is the president & CEO and board director at Deka. MPM BioImpact, Leaps by Bayer, Lumira Ventures, John Mumm, Echo Life Sciences, Viva BioInnovator, Samantha Connor, Plains VC, Alexandria Venture Investments, and AGP Ventures are investors in Deka. J.B.M. is an inventor on the following patents: “Dual cytokine fusion proteins comprising IL-10” (US11292822B2), “Dual cytokine fusions proteins comprising IL-10” (US11572397B2), “Methods for treating malignant tumors with IL-10 variant conjugates” (US10975133B2), and “Antibody variable domain regions fused to IL-10 variant molecules” (US10858412B2).
Figures






References
-
- Kiladjian J.J., Cassinat B., Chevret S., Turlure P., Cambier N., Roussel M., Bellucci S., Grandchamp B., Chomienne C., Fenaux P. Pegylated interferon-alfa-2a induces complete hematologic and molecular responses with low toxicity in polycythemia vera. Blood. 2008;112:3065–3072. doi: 10.1182/blood-2008-03-143537. - DOI - PubMed
-
- Charych D.H., Hoch U., Langowski J.L., Lee S.R., Addepalli M.K., Kirk P.B., Sheng D., Liu X., Sims P.W., VanderVeen L.A., et al. NKTR-214, an Engineered Cytokine with Biased IL2 Receptor Binding, Increased Tumor Exposure, and Marked Efficacy in Mouse Tumor Models. Clin. Cancer Res. 2016;22:680–690. doi: 10.1158/1078-0432.CCR-15-1631. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

