Redox mechanism of glycerophospholipids and relevant targeted therapy in ferroptosis
- PMID: 40744916
- PMCID: PMC12314118
- DOI: 10.1038/s41420-025-02654-y
Redox mechanism of glycerophospholipids and relevant targeted therapy in ferroptosis
Abstract
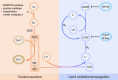
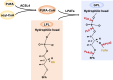
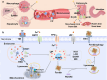
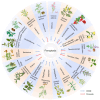
Ferroptosis, an iron-dependent form of regulated cell death driven by redox dysregulation, is defined by iron overload, reactive oxygen species overproduction, and subsequent peroxidation of polyunsaturated fatty acid-containing phospholipids, notably glycerophospholipids. This review comprehensively delineates the enzymatic such as lipoxygenases and non-enzymatic including Fenton reaction pathways governing glycerophospholipid peroxidation. Furthermore, we systematically dissect fine regulation of iron ions, including absorption, transport, and redox state transition. Given pathophysiological relevance of ferroptosis to numerous diseases, especially neurodegenerative disorders and various cancers, we evaluate emerging therapeutic strategies targeting key ferroptosis nodes, with a primary focus on the key enzymes involved in lipid peroxidation, transferrin receptor-mediated endocytosis mechanism and traditional Chinese medicine. Our work provides a direction for advancing ferroptosis research and developing combinatorial therapies that synergize ferroptosis induction with conventional treatments.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
Targeting ferroptosis in the treatment of ulcerative colitis by traditional Chinese medicine: A novel therapeutic strategies.Phytomedicine. 2025 Apr;139:156539. doi: 10.1016/j.phymed.2025.156539. Epub 2025 Feb 17. Phytomedicine. 2025. PMID: 39987602
-
Ferroptosis in health and disease.Redox Biol. 2024 Sep;75:103211. doi: 10.1016/j.redox.2024.103211. Epub 2024 May 30. Redox Biol. 2024. PMID: 38908072 Free PMC article. Review.
-
Salicylaldehyde Benzoylhydrazone Protects Against Ferroptosis in Models of Neurotoxicity and Behavioural Dysfunction, In Vitro and In Vivo.J Mol Neurosci. 2025 Jun 14;75(2):77. doi: 10.1007/s12031-025-02371-2. J Mol Neurosci. 2025. PMID: 40515790 Free PMC article.
-
The mist of ferroptosis: The Orpheus journey of mitochondria - Exploring the symphony of cell fate.Int J Biol Macromol. 2025 Aug;319(Pt 2):145472. doi: 10.1016/j.ijbiomac.2025.145472. Epub 2025 Jun 22. Int J Biol Macromol. 2025. PMID: 40555312 Review.
-
BNIP3-mediated mitophagy attenuates hypoxic-ischemic brain damage in neonatal rats by inhibiting ferroptosis through P62-KEAP1-NRF2 pathway activation to maintain iron and redox homeostasis.Acta Pharmacol Sin. 2025 Jan;46(1):33-51. doi: 10.1038/s41401-024-01365-x. Epub 2024 Aug 23. Acta Pharmacol Sin. 2025. PMID: 39179868
References
Publication types
LinkOut - more resources
Full Text Sources

