Retrospective study of onychomycosis patients treated with ciclopirox 8% HPCH and oral antifungals applying artificial intelligence to electronic health records
- PMID: 40744950
- PMCID: PMC12313871
- DOI: 10.1038/s41598-025-10875-5
Retrospective study of onychomycosis patients treated with ciclopirox 8% HPCH and oral antifungals applying artificial intelligence to electronic health records
Abstract
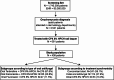
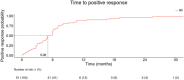
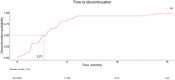
We conducted a multicenter retrospective analysis of 408 patients diagnosed with onychomycosis who attended three tertiary care Spanish hospitals. The study was conducted to assess the clinical characteristics and outcomes of onychomycosis patients undertaken combined treatment with ciclopirox 8% hydroxypropyl chitosan (CPX 8% HPCH) nail lacquer and oral antifungal agents (terbinafine, itraconazole, and fluconazole). Data were extracted and analyzed using the EHRead® technology based on natural language processing and machine learning and using SNOMED CT terminology. The mean age was 51.1 years, with 54.4% females. Repeated nail trauma was the most common risk factor (7.1%). More than half of the patients (56.6%) underwent fungal culture. Terbinafine, itraconazole, and fluconazole were used in 67.7%, 20.8%, and 11.5% of patients, respectively. Treatment synchronicity distribution revealed that 59.1% of patients started concomitant treatment with CPX 8% HPCH and oral antifungals, 27.9% had initial oral antifungals, and 13% had initial nail lacquer. The response to treatment (positive response 15.7%, presumed positive 59.8%) was unrelated to treatment synchronicity or type of antifungal agent. Erythema (5.6%), diarrhea (4.9%), and fever (4.2%) were the most frequently registered potential adverse events. These findings provide valuable insights for physicians regarding the management of patients with onychomycosis in daily practice.
Keywords: Artificial intelligence; Ciclopirox 8% HPCH; Fluconazole; Itraconazole; Machine learning; Onychomycosis; Real-world data; Terbinafine.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Institutional review board statement: Approval by the Ethics Committee of the participating hospitals was obtained.
Figures

References
-
- Gupta, A. K. et al. The prevalence of unsuspected onychomycosis and its causative organisms in a multicentre Canadian sample of 30 000 patients visiting physicians’ offices. J. Eur. Acad. Dermatol. Venereol.30, 1567–1572. 10.1111/jdv.13677 (2016). - PubMed
-
- Sigurgeirsson, B. & Baran, R. The prevalence of onychomycosis in the global population: a literature study. J. Eur. Acad. Dermatol. Venereol.28, 1480–1491. 10.1111/jdv.12323 (2014). - PubMed
-
- Gupta, A. K. et al. Prevalence and epidemiology of onychomycosis in patients visiting physicians’ offices: a multicenter Canadian survey of 15,000 patients. J. Am. Acad. Dermatol.43, 244–248. 10.1067/mjd.2000.104794 (2000). - PubMed
-
- Thomas, J. et al. Toenail onychomycosis: an important global disease burden. J. Clin. Pharm. Ther.35, 497–519. 10.1111/j.1365-2710.2009.01107.x (2010). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

