Enhancement of angiotensin II type 1 receptor-associated protein suppresses kidney inflammation in a mouse model of aristolochic acid nephropathy
- PMID: 40744957
- PMCID: PMC12314130
- DOI: 10.1038/s41598-025-08642-7
Enhancement of angiotensin II type 1 receptor-associated protein suppresses kidney inflammation in a mouse model of aristolochic acid nephropathy
Abstract
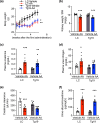
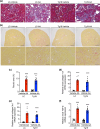
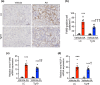
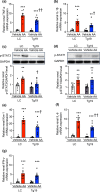
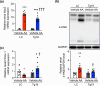
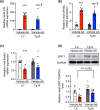
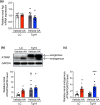
Chronic kidney disease generally progresses to irreversible fibrosis through chronic inflammation and age-related changes. We had previously reported that genetic knockdown of angiotensin II type 1 receptor (AT1R)-associated protein (ATRAP) exacerbates aging-associated kidney tubulointerstitial fibrosis in mice. However, whether enhanced ATRAP expression can suppress renal fibrosis and senescence in vivo remains unknown. Recently, we proposed that aristolochic acid nephropathy (AAN) could be used for modeling kidney aging with fibrosis. The present study aimed to investigate the functional role of ATRAP in aging-associated kidney fibrosis and inflammation using ATRAP transgenic (Tg19) mice subjected to AAN. AA administration caused histological renal fibrosis and enhanced ATRAP expression had no apparent effect on AA-induced renal fibrosis. However, enhanced ATRAP expression significantly suppressed AA-induced macrophage infiltration concomitant with reductions in inflammation-related, macrophage-related and senescence-related gene expression in the kidneys. Furthermore, the renal expression of anti-aging gene (Klotho, Sirtuin1) was significantly reduced in control mice in response to AA administration, whereas AA-mediated downregulation of Sirtuin1 expression was tendentially less prominent in Tg19 mice. Collectively, the enhancement of ATRAP expression failed to ameliorate renal fibrosis but partially attenuated renal inflammation and cellular senescence in AAN. Thus, ATRAP is a potential therapeutic target against renal inflammation and senescence.
Keywords: Angiotensin II type 1 receptor-associated protein; Anti-aging genes; Aristolochic acid nephropathy; Chronic kidney disease; Renal fibrosis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Effects of proximal tubule-specific ATRAP enhancement on hypertension in a remnant kidney chronic kidney disease model of mice.Sci Rep. 2025 Jul 28;15(1):27391. doi: 10.1038/s41598-025-12168-3. Sci Rep. 2025. PMID: 40721451 Free PMC article.
-
Angiotensin II Type 1 Receptor-Associated Protein Regulates Kidney Aging and Lifespan Independent of Angiotensin.J Am Heart Assoc. 2017 Jul 27;6(8):e006120. doi: 10.1161/JAHA.117.006120. J Am Heart Assoc. 2017. PMID: 28751545 Free PMC article.
-
Macrophage-derived exosomes promote telomere fragility and senescence in tubular epithelial cells by delivering miR-155.Cell Commun Signal. 2024 Jul 10;22(1):357. doi: 10.1186/s12964-024-01708-5. Cell Commun Signal. 2024. PMID: 38987851 Free PMC article.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers for adults with early (stage 1 to 3) non-diabetic chronic kidney disease.Cochrane Database Syst Rev. 2011 Oct 5;(10):CD007751. doi: 10.1002/14651858.CD007751.pub2. Cochrane Database Syst Rev. 2011. Update in: Cochrane Database Syst Rev. 2023 Jul 19;7:CD007751. doi: 10.1002/14651858.CD007751.pub3. PMID: 21975774 Updated.
References
-
- Cao, Q., Harris, D. C. & Wang, Y. Macrophages in kidney injury, inflammation, and fibrosis. Physiology30(3), 183–194 (2015). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

