Lacticaseibacillus rhamnosus attenuates uremic toxins in patients with nondialysis chronic kidney disease through the anti-inflammatory molecules
- PMID: 40744977
- PMCID: PMC12313892
- DOI: 10.1038/s41598-025-12768-z
Lacticaseibacillus rhamnosus attenuates uremic toxins in patients with nondialysis chronic kidney disease through the anti-inflammatory molecules
Abstract
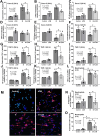
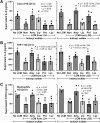
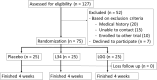
Because of the strain-dependent effect and the lack of simultaneous in vitro test with limited clinical data on Lacticaseibacillus rhamnosus L34 (L34) isolated from the Thai population, L34 was tested and compared with L. rhamnosus GG (LGG). The before and after test using L34 and a randomized placebo-controlled trial using placebo, L34, and LGG, for 4 weeks in patients with non-dialysis chronic kidney disease stage 3-5 (CKD) together with the in vitro experiments using indoxyl sulfate (IS, a representative uremic toxin) were performed. In comparison with the baseline, 4-week-L34 administration reduced gut-derived uremic toxins (GDUTs), except total IS, and attenuated several biomarkers, including i) systemic inflammation, as measured by cytokines and neutrophil extracellular traps using citrullinated histone 3, cell-free DNA, and fluorescent-stained nuclear morphology; ii) gut permeability defect (beta-D-glucan but not by endotoxemia); and iii) gut dysbiosis (fecal microbiome analysis). Additionally, L34-conditioned media attenuated IS-induced injuries on Caco-2 enterocytes, THP-1-derived-macrophages, and isolated neutrophils. Despite the possible different active compounds, both probiotics similarly attenuated IS-induced inflammation in vitro and in patients when compared with the placebo. In conclusion, L34 and LGG similarly attenuated systemic inflammation in patients with CKD, through the improved gut dysbiosis and anti-inflammation.
Keywords: Lacticaseibacillus rhamnosus; Chronic kidney disease; Gut leakage; Gut-derived uremic toxins; Probiotics.
© 2025. The Author(s).
Conflict of interest statement
Declaration. Competing interests: The authors declare no competing interests. Ethics approval: This study involves human participants and was approved by the research ethics board of the Chulalongkorn University Faculty of Medicine’s Institutional Review Board (IRB No. 0055/66). Participant gave informed consent to participate in the study before taking part. Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research..
Figures




Similar articles
-
Uremia-Induced Gut Barrier Defect in 5/6 Nephrectomized Mice Is Worsened by Candida Administration through a Synergy of Uremic Toxin, Lipopolysaccharide, and (1➔3)-β-D-Glucan, but Is Attenuated by Lacticaseibacillus rhamnosus L34.Int J Mol Sci. 2022 Feb 24;23(5):2511. doi: 10.3390/ijms23052511. Int J Mol Sci. 2022. PMID: 35269654 Free PMC article.
-
Synbiotics, prebiotics and probiotics for people with chronic kidney disease.Cochrane Database Syst Rev. 2023 Oct 23;10(10):CD013631. doi: 10.1002/14651858.CD013631.pub2. Cochrane Database Syst Rev. 2023. PMID: 37870148 Free PMC article.
-
Lactobacillus rhamnosus L34 attenuates chronic kidney disease progression in a 5/6 nephrectomy mouse model through the excretion of anti-inflammatory molecules.Nephrol Dial Transplant. 2022 Jul 26;37(8):1429-1442. doi: 10.1093/ndt/gfac032. Nephrol Dial Transplant. 2022. PMID: 35138387
-
Lactobacillus rhamnosus attenuates Thai chili extracts induced gut inflammation and dysbiosis despite capsaicin bactericidal effect against the probiotics, a possible toxicity of high dose capsaicin.PLoS One. 2021 Dec 23;16(12):e0261189. doi: 10.1371/journal.pone.0261189. eCollection 2021. PLoS One. 2021. PMID: 34941893 Free PMC article.
-
Impact of Gut Microbiome Modulation on Uremic Toxin Reduction in Chronic Kidney Disease: A Systematic Review and Network Meta-Analysis.Nutrients. 2025 Apr 3;17(7):1247. doi: 10.3390/nu17071247. Nutrients. 2025. PMID: 40219004 Free PMC article.
References
-
- Vaziri, N. D. et al. Chronic kidney disease alters intestinal microbial flora. Kidney Int.83(2), 308–315 (2013). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

