Fusion of SARS-CoV-2 neutralizing LCB1 peptide with Bacillus amyloliquefaciens RNase improves antiviral efficacy
- PMID: 40744985
- PMCID: PMC12313848
- DOI: 10.1038/s41598-025-12444-2
Fusion of SARS-CoV-2 neutralizing LCB1 peptide with Bacillus amyloliquefaciens RNase improves antiviral efficacy
Abstract
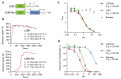
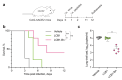
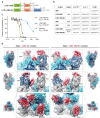
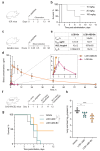
Virus-neutralizing peptides (VNPs) emerged as promising antiviral drug candidates with unprecedented specificity and cost-effectiveness during the recent COVID-19 pandemic. However, limited avidity, lack of effector functions, short circulatory half-life, and restricted administration routes make them inferior compared to neutralizing antibodies. To address these constraints, a potent VNP that targets the SARS-CoV-2 S protein is combined with Barnase, a highly active RNA-cleaving enzyme from Bacillus amyloliquefaciens. The resulting LCB1-Barnase (LCB1-Bn) chimera retains strong binding affinity for the SARS-CoV-2 S protein and demonstrates a fourfold reduction in IC50 compared to the LCB1 peptide alone in competitive ELISA and in in vitro neutralization tests. In transgenic CAG-hACE2 mice infected with wild-type SARS-CoV-2, intranasal administration of LCB1-Bn significantly improves survival and reduces viral load by 29-fold. To extend circulation life and allow systemic intravenous administration, an albumin-binding domain (ABD) from Streptococcus protein G is added to LCB1-Bn, producing LCB1-ABD-Bn fusion protein which displays a 95-fold increase in serum half-life. LCB1-ABD-Bn exhibits good tolerability at doses below 10 mg/kg and provides protection of SARS-CoV-2-infected CAG-hACE2 animals in 24-hour post-infection intraperitoneal treatment. Cryo-EM reveals the LCB1-ABD-Bn's tight interaction with S protein RBD domains, highlighting its potential as a promising drug candidate against SARS-CoV-2.
Keywords: Antiviral peptide; Barnase; Half-life extension; Pharmacokinetics; SARS-CoV-2; Virus neutralizing peptides (VNPs).
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures
References
-
- Quagliata, M., Papini, A. M. & Rovero, P. Chemically modified antiviral peptides against SARS-CoV-2. J Pept. Scie3541 (2023). - PubMed
-
- Shilova, O. N., Shilov, E. S. & Deyev, S. M. Design of targeted antiviral polypeptides specific to SARS-CoV-2. Challenges and prospects. Russ Chem. Rev.93, RCR5128 (2024).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

