Stoichiometric 14-3-3ζ binding promotes phospho-Tau microtubule dissociation and reduces aggregation and condensation
- PMID: 40745206
- PMCID: PMC12313985
- DOI: 10.1038/s42003-025-08548-0
Stoichiometric 14-3-3ζ binding promotes phospho-Tau microtubule dissociation and reduces aggregation and condensation
Abstract
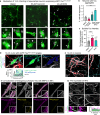
The microtubule (MT) association of protein Tau is decreased upon phosphorylation. Increased levels of phosphorylated Tau in the cytosol pose the risk of pathological aggregation, as observed in neurodegenerative diseases. We show that binding of 14-3-3ζ enhances cytosolic Tau solubility by promoting phosphorylated Tau removal from MTs, while simultaneously inhibiting Tau aggregation both directly and indirectly via suppression of condensate formation. These 14-3-3ζ activities depend on site-specific binding of 14-3-3 to Tau phosphorylated at S214 and S324. At sub-stoichiometric 14-3-3ζ concentrations, or in the presence of other 14-3-3ζ binding partners, multivalent electrostatic interactions promote Tau:14-3-3ζ co-condensation, offering a phosphorylation-independent mode of Tau-14-3-3ζ interactions. Given the high abundance of 14-3-3 proteins in the brain, 14-3-3 binding could provide efficient multi-modal chaperoning activity for Tau in the healthy brain and be important for preventing Tau aggregation in disease.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Christian Ottman and Luc Brunsveld are both co-founders of Ambagon Therapeutics. Christian Ottmann is currently employee and Luc Brunsveld is currently advisor of Ambagon Therapeutics.
Figures






References
-
- Khatoon, S., Grundke-Iqbal, I. & Iqbal, K. Brain levels of microtubule-associated protein τ are elevated in Alzheimer’s disease: a radioimmuno-slot-blot assay for nanograms of the protein. J. Neurochem.59, 750–753 (1992). - PubMed
-
- Goedert, M. Tau filaments in neurodegenerative diseases. FEBS Lett.592, 2383–2391 (2018). - PubMed
-
- Spillantini, M. G. & Goedert, M. Tau pathology and neurodegeneration. Lancet Neurol.12, 609–622 (2013). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

