Pantethine ameliorates dilated cardiomyopathy features in PPCS deficiency disorder in patients and cell line models
- PMID: 40745475
- PMCID: PMC12313872
- DOI: 10.1038/s43856-025-01017-z
Pantethine ameliorates dilated cardiomyopathy features in PPCS deficiency disorder in patients and cell line models
Abstract
Background: PPCS deficiency disorder (PPCS DD) is an ultra-rare, autosomal recessive form of dilated cardiomyopathy (DCM) caused by pathogenic variants in PPCS, which encodes the enzyme catalyzing the second step in the coenzyme A (CoA) biosynthesis pathway. To date, only six patients worldwide have been identified.
Methods: Whole-exome sequencing was performed to identify pathogenic PPCS variants in affected individuals. Protein stability was assessed by Western blotting. CoA levels were quantified using a microplate-based assay in patient-derived fibroblasts, cardiac progenitor cells, and cardiomyocytes. Functional evaluation of cardiac cells and engineered heart patches was conducted to investigate contractile performance and arrhythmogenicity. Pantethine was tested as a potential therapeutic agent both in vitro and through long-term clinical follow-up in patients.
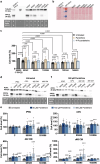
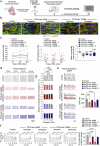
Results: Causative PPCS variants are identified in six individuals with DCM and variable associated features, including neuromuscular and neurological symptoms. Identified variants lead to reduced PPCS protein stability and decreased cellular CoA levels. Cardiac cells exhibit impaired contractility and arrhythmias, which are partially rescued by pantethine treatment. Clinically, patients receiving pantethine show sustained improvement over time.
Conclusions: Our study expands the genetic and clinical spectrum of PPCS deficiency disorder, identifying six new cases with diverse phenotypes. Functional investigations reveal reduced CoA levels and dysfunction in patient-derived cardiac cells. Pantethine treatment shows promise in partially rescuing DCM phenotypes, both in vitro and in patients. However, complete reversal may require early intervention. These findings underscore the importance of timely diagnosis and treatment in PPCS DD. Future research should focus on optimizing pantethine supplementation and exploring additional therapies to enhance CoA levels and cardiac function in affected individuals.
Plain language summary
PPCS deficiency disorder is an extremely rare inherited disease that causes heart muscle weakness (dilated cardiomyopathy) and other symptoms. It results from changes in a gene involved in making coenzyme A (CoA), a vital molecule for cell energy. This study identified six new patients with the condition and investigated how these gene changes affect heart function. Researchers used patient cells and lab-grown heart tissues to study the disease and tested pantethine, a compound that helps increase CoA levels. They found that pantethine improved heart cell function and showed positive effects in treated patients. These results highlight the importance of early diagnosis and treatment. In the future, therapies such as pantethine could offer hope for improving heart health in affected individuals.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Sibon serves as non-compensated executive for the Spoonbill Foundation and the Stichting Lepelaar, not-for-profit organizations that may benefit from the results of this research. All other authors declare that they have no competing interests.
Figures

Similar articles
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
PTS-Related Tetrahydrobiopterin Deficiency (PTPSD).2025 Jul 10. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. 2025 Jul 10. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. PMID: 40638773 Free Books & Documents. Review.
-
[Volume and health outcomes: evidence from systematic reviews and from evaluation of Italian hospital data].Epidemiol Prev. 2013 Mar-Jun;37(2-3 Suppl 2):1-100. Epidemiol Prev. 2013. PMID: 23851286 Italian.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
References
-
- Daugherty, M. et al. Complete Reconstitution of the Human Coenzyme A Biosynthetic Pathway via Comparative Genomics. J. Biol. Chem.277, 21431–21439 (2002). - PubMed
-
- Lok, A. et al. Novel phosphopantothenoylcysteine synthetase (PPCS) mutations with prominent neuromuscular features: Expanding the phenotypical spectrum of PPCS-related disorders. Am. J. Med. Genet. A. (2022) 10.1002/ajmg.a.62848. - PubMed
-
- Bravo‐Alonso, I. et al. Pathogenic variants of the coenzyme A biosynthesis‐associated enzyme phosphopantothenoylcysteine decarboxylase cause autosomal‐recessive dilated cardiomyopathy. J. Inherit. Metab. Dis. 12584 (2023) 10.1002/jimd.12584. - PubMed
-
- Bosveld, F. et al. De novo CoA biosynthesis is required to maintain DNA integrity during development of the Drosophila nervous system. Hum. Mol. Genet.17, 2058–2069 (2008). - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

