Aortic valve stenosis and osteoporosis: insights from a mouse model
- PMID: 40745526
- PMCID: PMC12312273
- DOI: 10.1186/s12872-025-05037-4
Aortic valve stenosis and osteoporosis: insights from a mouse model
Abstract
Background: Aortic valve stenosis (AVS) is the most common heart valve disease requiring intervention. Osteoporosis, affecting ~ 10% of those over 50, is linked to aortic valve calcification and increased AVS risk. However, its direct role in AVS development or progression remains unclear. Using a mouse model, we examined whether estrogen-deficiency-induced osteoporosis modifies early hemodynamic and histological remodeling of the aortic valve after mechanical injury, and whether zoledronic acid (ZA) treatment alters these processes.
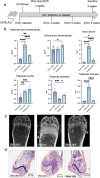
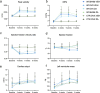
Methods: Osteoporosis was induced in mice via bilateral ovariectomy (OVX), with sham-operated controls. To prevent bone loss, ZA or vehicle were administered weekly via intraperitoneal injection. Two weeks post-OVX, AVS was induced by mechanically injuring the aortic valve under echocardiographic guidance, with control procedures (CTR) in separate groups. After model validation, C57BL/6J mice were assigned to six groups: WI Sham Vehicle, WI OVX Vehicle, WI OVX ZA, WI Sham ZA, CTR OVX Vehicle, and CTR OVX ZA. Echocardiography was performed at baseline and 2, 4, and 6 weeks post-injury. Bone density was assessed via micro-CT and histology, with peak aortic velocity as the primary endpoint. Mice were euthanized at 8 weeks for tissue analysis.
Results: OVX induced a significant reduction in trabecular bone mineral density (TBM, 50.6%, p = 0.0025). Treatment with ZA effectively reversed bone resorption in OVX mice (p < 0.0001) and even enhanced trabecular structures compared to sham-operated animals (increase of TBM by 130%, p < 0.0001). Mechanical injury to the aortic valve successfully induced AVS, as evidenced by increased peak velocity (1294 vs. 2157 mm/s, p < 0.0001) and mean pressure gradient 2 weeks post-procedure (1.58 vs. 4.19 mmHg, p < 0.0001). However, neither OVX nor ZA treatment influenced the severity of AVS. Histological analyses confirmed aortic valve thickening following injury. Picrosirius red and CD68 staining revealed no differences in collagen content or immune cell infiltration of the aortic valve between osteoporotic and non-osteoporotic animals.
Conclusion: OVX-induced osteoporosis did not affect AVS severity after mechanical injury in our study. This suggests osteoporosis may not directly influence AVS in the early, pre-calcific stage that was studied in this model. However, to overcome limitations of the study, further studies with longer durations or refined models are needed to confirm these findings.
Keywords: Animal model; Aortic valve stenosis; Heart valve disease; Osteoporosis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All animal procedures were conducted in accordance with institutional and national guidelines for the care and use of laboratory animals. The study protocol was approved by the Landesamt für Natur, Umwelt und Verbraucherschutz Nordrhein-Westfalen (LANUV) under the approval number TV 81-02.04.2022.A042. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests. Clinical trial number: Not applicable.
Figures

References
-
- Goody PR, Hosen MR, Christmann D, Niepmann ST, Zietzer A, Adam M, et al. Aortic valve stenosis: from basic mechanisms to novel therapeutic targets. Arterioscler Thromb Vasc Biol. 2020;40(4):885–900. - PubMed
-
- Dweck MR, Jenkins WSA, Vesey AT, Pringle MAH, Chin CWL, Malley TS et al. 18F-Sodium Fluoride uptake is a marker of active calcification and disease progression in patients with aortic stenosis. Circulation. 2014;7(2):371–8. - PubMed
-
- Otto CM, Kuusisto J, Reichenbach DD, Gown AM, O’Brien KD. Characterization of the early lesion of ‘degenerative’ valvular aortic stenosis. Histological and immunohistochemical studies. Circulation. 1994;90(2):844–53. - PubMed
-
- Pawade TA, Newby DE, Dweck MR. Calcification in aortic stenosis: the skeleton key. J Am Coll Cardiol. 2015;66(5):561–77. - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

