Association of T-Cell Profiles With Disease Severity, Drug-Induced Liver Injury, and Treatment Completion in Tuberculosis
- PMID: 40746087
- PMCID: PMC12314307
- DOI: 10.1111/crj.70114
Association of T-Cell Profiles With Disease Severity, Drug-Induced Liver Injury, and Treatment Completion in Tuberculosis
Abstract
Background: Tuberculosis (TB) treatment is challenged by a long duration, poor adherence, and the high risk of drug-induced liver injury (DILI). T-cell immunity is essential for anti-mycobacterial defense, but current immune-monitoring methods poorly reflect disease severity and treatment response. Correlations of immune subpopulations with TB severity, DILI, and treatment prognosis remain poorly understood.
Methods: Peripheral blood mononuclear cells were collected from confirmed TB patients (n = 40). Multiparameter flow cytometry analysis was used to assess previously defined TB-associated T-cell phenotypes based on the co-expression of cytokines and immune checkpoint molecules following stimulation with two Mycobacterium tuberculosis peptides: culture filtrate protein 10 and early secreted antigenic target 6. Patients were subgrouped by disease severity, DILI, and treatment regimen (16-week short course vs. 24-week standard).
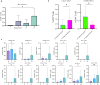
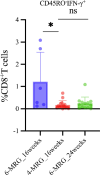
Results: Specific subsets (14/124) were found to be associated with disease severity. Notably, six of 14 subsets were positive for programmed death-ligand 1 (PD-L1), indicating its potential role in disease progression. DILI was associated with three interleukin (IL)-21+ subsets (naïve CD4+, memory CD8+, and interferon [IFN]-γ- CD4+ T cells) and IL-17+ memory CD8+ T cells, along with PD-L1+TIM-3+CD4+ T cells (all p < 0.05). The 16-week and 24-week treatment groups showed a significant difference in IFN-γ+ naïve CD8+ T cells at week 16 (p = 0.013), but not at treatment completion (p = 0.393), despite the different durations.
Conclusions: This study identifies specific T-cell phenotypes associated with TB severity, DILI, and treatment dynamics, highlighting potential immune markers for disease monitoring and DILI prediction.
Keywords: T‐cell phenotypes; drug‐induced liver injury; immune checkpoint molecules; treatment response; tuberculosis.
© 2025 The Author(s). The Clinical Respiratory Journal published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Immunogenicity, safety, and efficacy of the vaccine H56:IC31 in reducing the rate of tuberculosis disease recurrence in HIV-negative adults successfully treated for drug-susceptible pulmonary tuberculosis: a double-blind, randomised, placebo-controlled, phase 2b trial.Lancet Infect Dis. 2025 Jul;25(7):751-763. doi: 10.1016/S1473-3099(24)00814-4. Epub 2025 Mar 5. Lancet Infect Dis. 2025. PMID: 40056922 Clinical Trial.
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
Comprehensive single-cell chromatin and transcriptomic profiling of peripheral immune cells in nonsegmental vitiligo.Br J Dermatol. 2025 Jun 20;193(1):115-124. doi: 10.1093/bjd/ljaf041. Br J Dermatol. 2025. PMID: 39888372
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
References
-
- World Health Organization , Global Tuberculosis Report, 2023 (World Health Organization, 2023).
-
- Gunther G., Heyckendorf J., Zellweger J. P., et al., “Defining Outcomes of Tuberculosis (Treatment): From the Past to the Future,” Respiration 100, no. 9 (2021): 843–852. - PubMed
-
- World Health Organization , “WHO Consolidated Guidelines on Tuberculosis,” in Module 3: Diagnosis (World Health Organization, 2025). - PubMed
MeSH terms
Substances
Grants and funding
- 2023YFC2307300/the National Key R&D Program of China
- GWVI-9 and GWVI-11.1-05/Shanghai three-year (2023-2025) action plan to strengthen the public health system
- GWVI-11.2-YQ06/Shanghai three-year (2023-2025) action plan to strengthen the public health system
- 2018ZX10301/Multi-Dimensional Diagnostic Strategies for Tuberculosis and R&D of Innovative Products Program
LinkOut - more resources
Full Text Sources
Medical
Research Materials

