Anticancer and Anti-Inflammatory Effects of Benzothiazole Derivatives Targeting NF-κB/COX-2/iNOS in a Hepatocellular Carcinoma Cell Line
- PMID: 40746246
- PMCID: PMC12316066
- DOI: 10.4274/tjps.galenos.2025.49840
Anticancer and Anti-Inflammatory Effects of Benzothiazole Derivatives Targeting NF-κB/COX-2/iNOS in a Hepatocellular Carcinoma Cell Line
Abstract
Objectives: Benzothiazole compounds, characterized by their diverse biological and pharmacological properties, have emerged as promising molecules for suppressing cancer cell proliferation and invasion due to their antiproliferative attributes. Prior research from our laboratory revealed that 2-substituted benzothiazole compounds inhibit the proliferation of glioma and cervical cancer cells and induce apoptosis in pancreatic cancer cells. However, there is limited research on the effectiveness of benzothiazoles against hepatocellular carcinoma cells (HCC). This study sought to elucidate the anticancer potential of 2-substituted benzothiazole derivatives through their modulation of oxidative stress and inflammation mediators.
Materials and methods: Antiproliferative effects of two-step synthesized 2-substituted benzothiazole derivatives were evaluated on HepG2 cells via MTT assay. Apoptosis induction was assessed using Annexin V/PI staining; cell cycle arrest effects were determined through cell cycle analysis; cell migration was examined via wound healing assay; and mitochondrial membrane damage was quantified using JC-1 staining. Spectrophotometric measurements of total antioxidant status (TAS), total oxidant status, superoxide dismutase (SOD), total thiol, and native thiol levels were used to assess cellular redox status. Expression of nuclear factor kappa B (NF-κB), an inflammatory marker, was assessed by western blot, while inflammation-related cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase levels were measured using ELISA.
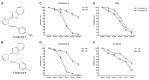
Results: This investigation unveiled benzothiazole derivatives' antiproliferative and cytotoxic properties against HepG2 cells (IC50 values of 56.98 μM and 59.17 μM at 24 h, and 38.54 μM 29.63 at 48 h). The synthesized compounds exhibited the ability to suppress cell migration and induce apoptosis, mediated by mitochondrial membrane potential loss (wound‑closure rates of 84.0 and 90.4% vs. 51.7% control at 48 h, apoptosis rates of 10.70% and 45.22% vs. 1.02% control). Furthermore, these derivatives reduced SOD activity (A and B at 100 μM p<0.001), TAS levels (A and B at 100 μM, p < 0.05, p < 0.001), and dynamic disulfide content. Notably, a decrease in NF-κB protein levels, closely associated with inflammation, was observed, along with a subsequent reduction in downstream effectors COX-2 (A and B at 100 μM, p<0.001) and iNOS (A and B at 100 μM, p < 0.001).
Conclusion: The findings of this study underscore the antiproliferative effects of benzothiazole derivatives in human HCCs, coupled with their anti-inflammatory potential by diminishing NF-κB levels.
Keywords: Benzothiazole; cyclooxygenase-2; inducible nitric oxide synthase; inflammation; nuclear factor kappa B; oxidative stress.
Copyright© 2025 The Author. Published by Galenos Publishing House on behalf of Turkish Pharmacists’ Association.
Conflict of interest statement
Conflict of Interest: The authors declare no conflicts of interest.
Figures





Similar articles
-
Buzhong Yiqi decoction improves inflammation and oxidative damage in autoimmune thyroiditis by inhibiting apoptosis via the SIRT1-Mediated Nrf2/NF-κB axis.J Ethnopharmacol. 2025 Jul 24;351:119967. doi: 10.1016/j.jep.2025.119967. Epub 2025 May 11. J Ethnopharmacol. 2025. PMID: 40360040
-
Commiphora leptophloeos leaf and bark extracts modulate OxInflammation through TLR4/ NF-κB/ Nrf2 pathways.J Ethnopharmacol. 2025 Jul 31;353(Pt A):120347. doi: 10.1016/j.jep.2025.120347. Online ahead of print. J Ethnopharmacol. 2025. PMID: 40752598
-
Investigation of novel nimesulide derivatives against breast cancer.Bioorg Chem. 2025 Aug 6;164:108850. doi: 10.1016/j.bioorg.2025.108850. Online ahead of print. Bioorg Chem. 2025. PMID: 40780124
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
