Proteomic and structural comparison between cilia from primary ciliary dyskinesia patients with a DNAH5 defect
- PMID: 40746421
- PMCID: PMC12310455
- DOI: 10.3389/fmolb.2025.1593810
Proteomic and structural comparison between cilia from primary ciliary dyskinesia patients with a DNAH5 defect
Abstract
Introduction: Primary ciliary dyskinesia (PCD) is a genetic disorder affecting motile cilia across various organs, leading to recurrent respiratory infections, subfertility, and laterality defects. While several diagnostic tools exist-such as high-speed video microscopy, immunofluorescence staining, electron microscopy, and genetic screening-the relationship between different pathogenic variants within a single PCD gene and their effects on ciliary composition, structure, and clinical phenotype remains poorly understood.
Methods: To investigate this, we analyzed cilia from PCD patients with different mutations in axonemal dynein heavy chain dnah5 using mass spectrometry and cryo-electron tomography. These methods allowed us to examine both the protein composition and ultrastructural organization of motile cilia in affected individuals.
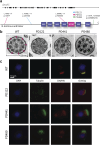
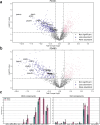
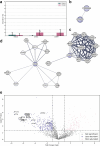
Results: Though all analyzed patients present similarly in traditional diagnostic methods, we observed differences in axonemal composition among patients carrying different dnah5 mutations. Specific reductions in ciliary components varied between individuals, indicating a mutation-specific impact. Notably, proteins such as VWA3B, KIAA1430/CFAP97, and DTHD1-not previously identified as components of human respiratory motile cilia-were detected in wild type cilia, but not in patient cilia. Lastly, we confirmed some changes in protein abundance in the 96-nm repeated unit of the axoneme between wild-type and PCD samples.
Discussion: These findings suggest that mutations in dnah5 result in varied and specific alterations in axonemal composition, reflecting the heterogeneity of the disease at the molecular level. The discovery of novel ciliary proteins and mutation-specific differences enhances our understanding of the complexity of PCD pathogenesis and may inform future diagnostic and therapeutic strategies.
Keywords: DNAH5; axoneme; cryo-electron tomography; dynein; mass spectrometry; primary ciliary dyskinesia.
Copyright © 2025 de Ceuninck van Capelle, Luo, Leitner, Tschanz, Latzin, Ott, Herren, Müller and Ishikawa.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Molecular Insights into Outer Dynein Arm Defects in Primary Ciliary Dyskinesia: Involvement of ZMYND10 and GRP78.Cells. 2025 Jun 17;14(12):916. doi: 10.3390/cells14120916. Cells. 2025. PMID: 40558543 Free PMC article.
-
Clinical, Genetic, Morphological and Functional Correlations in a Large Series of Patients with Primary Ciliary Dyskinesia: A Heterogeneous Disease with a Controversial Diagnosis.Mol Diagn Ther. 2025 Jul 31. doi: 10.1007/s40291-025-00801-w. Online ahead of print. Mol Diagn Ther. 2025. PMID: 40742517
-
The Association of Neonatal Respiratory Distress With Ciliary Ultrastructure and Genotype in Primary Ciliary Dyskinesia.Pediatr Pulmonol. 2025 May;60(5):e71091. doi: 10.1002/ppul.71091. Pediatr Pulmonol. 2025. PMID: 40344341 Free PMC article.
-
Clinical and genetic spectrum of primary ciliary dyskinesia in Chinese patients: a systematic review.Orphanet J Rare Dis. 2022 Jul 19;17(1):283. doi: 10.1186/s13023-022-02427-1. Orphanet J Rare Dis. 2022. PMID: 35854386 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
References
LinkOut - more resources
Full Text Sources

