Unconventional T cells in anti-cancer immunity
- PMID: 40746558
- PMCID: PMC12310680
- DOI: 10.3389/fimmu.2025.1618393
Unconventional T cells in anti-cancer immunity
Abstract
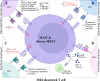
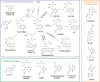
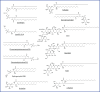
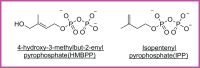
Unlike conventional T cells that detect peptide antigens loaded to major histocompatibility complex (MHC) molecules, unconventional T cells respond to non-peptidic metabolite antigens presented by MHC class I-like proteins, such as CD1 and MHC-related protein 1 (MR1). Semi-invariant mucosal-associated invariant T (MAIT) cells, γδ T cells, and invariant natural killer T (iNKT) cells, together with other CD1- or MR1-restricted T cell subsets expressing diverse T cell receptors (TCR), elicit an innate-like response independent of diverse MHC genetics. In contrast to an overall enhanced response to bacterial-derived riboflavin precursor metabolites in infections, MAIT cells often exhibit an immunosuppressive or exhausted phenotype in glioblastoma, lung cancer, colorectal cancer, and various hematological malignancies. Whereas some tumor cells can activate MAIT cells, the structures and functions of tumor-derived MR1 ligands remain largely unknown. Novel discoveries of mammalian-derived agonists and antagonists binding to MR1 protein are our knowledge of MR1 ligand structures and functions from MAIT cell activation in healthy conditions to anti-cancer immunity. Recent findings reveal that nucleoside and nucleobase analogs, as self-metabolites to activate MR1-restricted T cells, are regulated in the tumor microenvironment. Likewise, iNKT cells exhibit a dynamic role in cancer, capable of both protumor and antitumor immunity. Similarly, γδ T cells have also demonstrated both protective and tumor-promoting roles, via recognizing stress-induced protein and metabolite ligands. This review further depicts the distinct kinetics of responses, highlighting a rapid activation of unconventional T cells in solid versus hematological cancers. Emerging therapeutic strategies, including antigen-loaded MR1 and CD1, adoptive T cell transfer, chimeric antigen receptor-T (CAR-T) cells, T cell receptor-T (TCR-T) cells, and combination treatments with immune checkpoint inhibitors, yet remain challenging, hold promise in overcoming tumor-induced immunosuppression and genetic restriction of conventional T cell therapies. By addressing critical gaps, such as novel structures and functions of cancer metabolite antigens, unconventional T cells offer unique advantages in anti-cancer immunotherapy.
Keywords: CD1; MHC class I-related protein 1 (MR1); cancer; immunotherapy; lipids; polar metabolites; unconventional T cells.
Copyright © 2025 Laub, Rodrigues de Almeida and Huang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


Similar articles
-
Signals that control MAIT cell function in healthy and inflamed human tissues.Immunol Rev. 2024 May;323(1):138-149. doi: 10.1111/imr.13325. Epub 2024 Mar 22. Immunol Rev. 2024. PMID: 38520075 Free PMC article. Review.
-
Mouse mucosal-associated invariant T cell receptor recognition of MR1 presenting the vitamin B metabolite, 5-(2-oxopropylideneamino)-6-d-ribitylaminouracil.J Biol Chem. 2024 May;300(5):107229. doi: 10.1016/j.jbc.2024.107229. Epub 2024 Mar 25. J Biol Chem. 2024. PMID: 38537698 Free PMC article.
-
Diversity and hallmarks of metabolites surveyed by MR1.Immunobiology. 2025 Jul;230(4):153091. doi: 10.1016/j.imbio.2025.153091. Epub 2025 Jun 27. Immunobiology. 2025. PMID: 40651431 Review.
-
The MR1/MAIT cell axis impacts the gut-brain axis through both cognition and microbial community structure in 5XFAD mice.Alzheimers Dement. 2025 Jul;21(7):e70493. doi: 10.1002/alz.70493. Alzheimers Dement. 2025. PMID: 40696831 Free PMC article.
-
Disruption of riboflavin biosynthesis in mycobacteria establishes riboflavin pathway intermediates as key precursors of MAIT cell agonists.PLoS Pathog. 2025 Jul 1;21(7):e1012632. doi: 10.1371/journal.ppat.1012632. eCollection 2025 Jul. PLoS Pathog. 2025. PMID: 40591719 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

