Formal [1 + 2 + 3] Annulation of Anilines and CF3‑Containing Ynones via 6π-Electrocyclization
- PMID: 40746583
- PMCID: PMC12308597
- DOI: 10.1021/prechem.5c00037
Formal [1 + 2 + 3] Annulation of Anilines and CF3‑Containing Ynones via 6π-Electrocyclization
Abstract
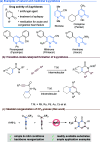
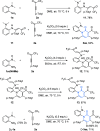
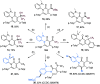
Six-membered N-heterocycles, such as 2-pyridones, are crucial in bioactive compounds and prevalent in natural products and pharmaceuticals, necessitating innovative synthesis approaches. Traditional methods, typically reliant on the transition-metal-catalyzed direct cyclization of alkynes, face limitations in product complexity. This study introduces a [1 + 2 + 3] annulation strategy for synthesizing 2-pyridones, employing anilines and CF3-ynones through a base-promoted metal-free catalytic system. This method offers a more streamlined approach to generating polysubstituted 2-pyridones, demonstrating enhanced functional group compatibility across substrates compared with existing transformations. The anilines' adjacent dialkyl amino groups significantly contribute to the reaction, serving as both proton reservoirs and directing groups, facilitating the formation of 2-pyridones. This reaction involves a ring closure/opening sequence, followed by aza-6π-electrocyclization and a C-C bond cleavage-driven aromatization process. The method's synthetic utility is further validated by its applicability in subsequent transformations, marking an advancement in the synthesis of complex N-heterocyclic compounds.
Keywords: 2-Pyridones; Anilines; CF3-containing ynones; Metal free; [1 + 2 + 3] annulation.
© 2025 The Authors. Co-published by University of Science and Technology of China and American Chemical Society.
Figures
References
-
- Torres M., Gil S., Parra M.. New Synthetic Methods to 2-Pyridone Rings. Curr. Org. Chem. 2005;9:1757–1779. doi: 10.2174/138527205774610886. - DOI
-
- Park N. H., Shin K. H., Kang M. K.. 34-Antifungal and Antiviral Agents. Pharmacol. Ther. Dent. 2017:488–503. doi: 10.1016/B978-0-323-39307-2.00034-5. - DOI
-
- Mathew R., DiSanto P., Jung R. G., Marbach J. A., Hutson J., Simard T., Ramirez F. D., Harnett D. T., Merdad A., Almufleh A., Weng W., Abdel-Razek O. S., Fernando M., Kyeremanteng K., Bernick J., Wells G. A., Chan V., Froeschl M., Labinaz M., Le May M. R., Russo J. J., Hibbert B.. Milrinone as Compared with Dobutamine in the Treatment of Cardiogenic Shock. New Eng. J. Med. 2021;385:516–525. doi: 10.1056/NEJMoa2026845. - DOI - PubMed
-
- Falb E., Ulanenko K., Tor A., Gottesfeld R., Weitman M., Afri M., Gottlieb H., Hassner A.. A highly efficient Suzuki-Miyaura methylation of pyridines leading to the drug pirfenidone and its CD3 version (SD-560) Green Chem. 2017;19:5046–5053. doi: 10.1039/C7GC01740E. - DOI
LinkOut - more resources
Full Text Sources