Development of a one-pot RPA-cas12a/13a assay for simultaneous detection of HPV16 and HPV18
- PMID: 40746592
- PMCID: PMC12310682
- DOI: 10.3389/fbioe.2025.1608301
Development of a one-pot RPA-cas12a/13a assay for simultaneous detection of HPV16 and HPV18
Abstract
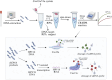
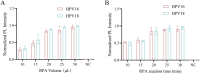
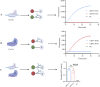
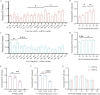
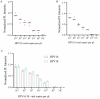
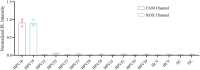
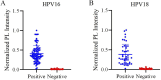
The incidence of human papillomavirus (HPV)-related cervical cancers has been on the rise, and the affected population is increasingly younger. Early-stage prevention and screening initiatives have emphasized the critical necessity for reliable and rapid HPV detection technique. In this study, we devised a fluorescence-based assay that integrated one-pot Cas12a/13a with recombinase polymerase amplification (RPA) for the detection of HPV16 and HPV18. We exploited the cleavage activities of the Cas12a and Cas13a enzymes to specifically target the L1 gene of HPV16 and 18, respectively. The diagnostic efficacy of the CRISPR-Cas12a/13a system was assessed in identifying HPV by analyzing clinical samples and comparing it with the PCR method. The one-pot RPA-Cas12a/13a-based fluorescence assays exhibited a sensitivity of 10 copies/µL, and required only 40 min for completion. Compared with PCR method, the overall sensitivity and specificity of this assay were 97.69% and 100%, respectively, with a kappa value of 0.967. This study presents a novel approach for cervical cancer screening and HPV infection surveillance, which may hold potential for the early diagnosis and prevention of HPV-related cervical malignancies.
Keywords: CRISPR-Cas12a/13a; HPV; multiplex detection; one-pot reaction; recombinase polymerase amplification.
Copyright © 2025 Yu, Luo, Huang, An, Wang, Chen, Rong, Zhang, Huang, Rao and Zha.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Cai S., Miao K., Tan X. Y., Cheng S., Li D. T., Zeng X. Y., et al. (2022). Clinical research progress and implications of therapeutic vaccines for cervical cancer and precancerous lesions: a qualitative systematic review. Zhonghua Zhong Liu Za Zhi 44, 743–760. 10.3760/cma.j.cn112152-20210824-00638 - DOI - PubMed
LinkOut - more resources
Full Text Sources

