Proactive Therapeutic Drug MONiToring to Guide Suppressive Antibiotic Therapy with DALBAvaNcin ( > 12 weeks) in Osteoarticular Infections (MONTALBANO)
- PMID: 40746661
- PMCID: PMC12311387
- DOI: 10.5194/jbji-10-255-2025
Proactive Therapeutic Drug MONiToring to Guide Suppressive Antibiotic Therapy with DALBAvaNcin ( > 12 weeks) in Osteoarticular Infections (MONTALBANO)
Abstract
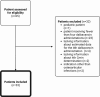
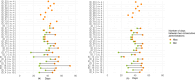
Introduction: Long-term dalbavancin use is increasingly adopted off-label for osteoarticular infections (OAIs), but data on administration timing and long-term effects beyond 12 weeks are scarce. This study evaluated the pharmacological efficacy of proactive therapeutic drug monitoring (TDM) to optimize dalbavancin administration. Methods: This single-center, retrospective study included adult OAI patients treated with doses of dalbavancin from July 2022 to October 2024. Initial doses were given on days 1, 8, and 43. From the third dose onward, and values informed dosing schedules via log-linear regression models, targeting mg L-1. The primary outcome was the pharmacological efficacy of dalbavancin, assessed by the proportion of patients with mg L-1 and mg L-1 after the third dose. Clinical outcomes and safety data were collected as descriptive data. Results: A total of 33 patients provided 118 determinations. Pharmacological efficacy was achieved in 93 118 (78.8 %) and 114 118 (96.6 %) determinations for thresholds of mg L-1 and mg L-1, respectively. Efficacy improved when considering only determinations at the correct timing. A total of 18 (54.5 %) patients are still in treatment, while 11 (33.3 %) completed therapy with clinical success. Three patients experienced a relapse after the end of the treatment, while one patient experienced failure, and no adverse events were reported. Conclusions: Dalbavancin is a viable option for prolonged OAI management when other therapies are unavailable or high-risk. Proactive TDM effectively supports this approach by ensuring adequate drug exposure while preventing accumulation.
Copyright: © 2025 Chiara Mariani et al.
Conflict of interest statement
Andrea Gori received a fee from Janssen, ViiV, MSD, BMS, Abbvie, Gilead, Novartis, Pfizer, Astellas, Astrazeneca, and Angelini. Dario Cattaneo received a speaker's fee from Pfizer, MSD, and Gilead and educational grants from ViiV Healthcare. However, all the authors declare that they do not have known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Etanercept and efalizumab for the treatment of psoriasis: a systematic review.Health Technol Assess. 2006 Nov;10(46):1-233, i-iv. doi: 10.3310/hta10460. Health Technol Assess. 2006. PMID: 17083854
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Rituximab for myasthenia gravis.Cochrane Database Syst Rev. 2025 Jul 3;7(7):CD014574. doi: 10.1002/14651858.CD014574.pub2. Cochrane Database Syst Rev. 2025. PMID: 40607605 Review.
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Effect of body mass index on the timing of therapeutic drug monitoring-guided dalbavancin dosing in patients with osteoarticular infections.J Antimicrob Chemother. 2025 Jun 3;80(6):1726-1732. doi: 10.1093/jac/dkaf132. J Antimicrob Chemother. 2025. PMID: 40256850
References
-
- Azamgarhi T, Warren S, Scobie A, Karunaharan N, Perez-Sanchez C, Houghton R, Hassan S, Lourtet-Hascoët J, Kershaw H, Sendi P, Saeed K. Dalbavancin to facilitate early discharge in the treatment of complex musculoskeletal infections: a multi-centre real-life application. J Bone Joint Infect. 2025;10:93–100. doi: 10.5194/jbji-10-93-2025. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources