The efficacy and safety of cabazitaxel in the treatment of metastatic castration-resistant prostate cancer: a systematic review and network meta-analysis based on randomized controlled trials
- PMID: 40746720
- PMCID: PMC12310731
- DOI: 10.3389/fphar.2025.1586650
The efficacy and safety of cabazitaxel in the treatment of metastatic castration-resistant prostate cancer: a systematic review and network meta-analysis based on randomized controlled trials
Abstract
Background: Cabazitaxel (CAB) has been approved for the treatment of patients with progressed metastatic castration-resistant prostate cancer (mCRPC) after receiving docetaxel. To assess the efficacy and safety of CAB in mCRPC patients through systematic review and network meta-analysis.
Methods: Randomized controlled studies on the treatment of mCRPC with CAB in PUBMED, EMBASE, Cochrane, and Web of Science were searched. Relevant studies that met pre-set criteria were determined, and the quality of included studies was evaluated using the National Institutes of Health-Quality Assessment Tool. After the data was extracted, data analysis was conducted in R 4.3.2. Overall survival (OS), progression-free survival (PFS), and serious adverse events (SAEs) were used as the primary outcomes, and HR (hazard ratio) or RR (risk ratio) and their 95% confidence intervals (95% CrI) were calculated as effect sizes.
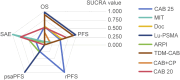
Results: A total of 13 studies were included, involving 5,814 patients. The overall risk of bias for 13 studies was low. The results showed that CAB 25 mg/m2 significantly improved OS compared to androgen receptor pathway inhibitor (ARPI) (1.50 [1.30, 1.70]) and MIT (1.40 [1.20, 1.70]), but its efficacy was inferior to Lu-PSMA (0.42 [0.27, 0.67]) and therapeutic drug monitoring (TDM)-CAB (0.51 [0.38, 0.70]). CAB 25 mg/m2 could significantly improve PFS compared to CAB + CP (1.40 [1.10, 2.00]), ARPI (1.80 [1.50, 2.30], MIT (1.40 [1.20, 1.60]), but its efficacy was not as good as CAB 20 mg/m2 (0.92 [0.82, 1.00]), Lu-PSMA (0.58 [0.41, 0.80]), TDM-CAB (0.67 [0.51, 0.86]). In addition, compared to CAB 25 mg/m2, CAB + CP may significantly increase the risk of SAEs (3.10 [1.70, 5.90]).
Conclusion: CAB is an effective treatment in mCRPC, and combining it with other treatment methods may enhance efficacy, but attention should be paid to the occurrence of adverse events.
Keywords: cabazitaxel; castration resistance; meta-analysis; prostate cancer; systematic review.
Copyright © 2025 Shao, Wan, Guo and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
First-line therapy for adults with advanced renal cell carcinoma: a systematic review and network meta-analysis.Cochrane Database Syst Rev. 2023 May 4;5(5):CD013798. doi: 10.1002/14651858.CD013798.pub2. Cochrane Database Syst Rev. 2023. PMID: 37146227 Free PMC article.
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
References
-
- Annala M., Fu S., Bacon J. V. W., Sipola J., Iqbal N., Ferrario C., et al. (2021). Cabazitaxel versus abiraterone or enzalutamide in poor prognosis metastatic castration-resistant prostate cancer: a multicentre, randomised, open-label, phase II trial. Ann. Oncol. 32 (7), 896–905. 10.1016/j.annonc.2021.03.205 - DOI - PubMed
-
- Baciarello G., Delva R., Gravis G., Tazi Y., Beuzeboc P., Gross-Goupil M., et al. (2022). Patient preference between cabazitaxel and docetaxel for first-line chemotherapy in metastatic castration-resistant prostate cancer: the CABADOC trial. Eur. Urol. 81 (3), 234–240. 10.1016/j.eururo.2021.10.016 - DOI - PubMed
-
- Beer T. M., Hotte S. J., Saad F., Alekseev B., Matveev V., Fléchon A., et al. (2017). Custirsen (OGX-011) combined with cabazitaxel and prednisone versus cabazitaxel and prednisone alone in patients with metastatic castration-resistant prostate cancer previously treated with docetaxel (AFFINITY): a randomised, open-label, international, phase 3 trial. Lancet Oncol. 18 (11), 1532–1542. 10.1016/s1470-2045(17)30605-8 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous