Regioisomeric Control of Charge Transport Properties in Fluoranthene-Fused 12-Ring Heteroarenes
- PMID: 40747029
- PMCID: PMC12308440
- DOI: 10.1021/jacsau.5c00503
Regioisomeric Control of Charge Transport Properties in Fluoranthene-Fused 12-Ring Heteroarenes
Abstract
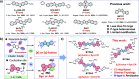
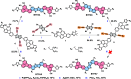
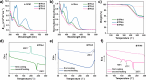
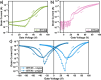
Heteroarene-based organic semiconductors (OSCs) have emerged as promising material candidates for large-area, flexible electronic and photonic devices due to their favorable π-conjugation systems and tunable optoelectronic properties. However, their development is still hindered by synthetic and design challenges, in particular, the limited access to highly polycyclic heteroarenes with tunable properties and the unexplored effects of topological isomerism. Here, we have successfully synthesized three regioisomeric thienoacenes (BTFA4-6) by fusing a dicyanofluoranthene unit, which have an identical 12-fused ring composition but different molecular topologies with [a] or [c]-fusion and syn or anti-CN substitution. We demonstrate that solution-processable BTFA4-6 exhibits topology-dependent charge transport properties by fabricating organic field-effect transistors. BTFA4 with [a]-fusion and syn-CN substitution and BTFA6 with [c]-fusion and syn-CN substitution show only typical n-type semiconducting behavior, with electron mobilities of 3.91 × 10-4 and 1.58 × 10-5 cm2 V-1 s-1, respectively, while BTFA5 with [a]-fusion and anti-CN substitution achieves electron-dominated ambipolar behavior, with an increase in electron mobility (3.45 × 10-2 cm2 V-1 s-1) by 2 orders of magnitude and a hole mobility of 4.31 × 10-3 cm2 V-1 s-1. Therefore, this work establishes that regioisomeric molecular engineering is a powerful tool for manipulating the charge transport properties of heteroarene-based OSCs.
Keywords: Charge transport property; Heteroarene; Organic semiconductor; Regioisomerism.
© 2025 The Authors. Published by American Chemical Society.
Figures


Similar articles
-
Molecular Gridization of Organic Semiconducting π Backbones.Acc Chem Res. 2025 Jul 1;58(13):1982-1996. doi: 10.1021/acs.accounts.5c00180. Epub 2025 Jun 13. Acc Chem Res. 2025. PMID: 40512962
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
Two-Dimensional Layered Simple Aliphatic Monoammonium Tin Perovskite Thin Films and Potential Applications in Field-Effect Transistors.ACS Appl Mater Interfaces. 2022 Nov 9;14(44):50401-50413. doi: 10.1021/acsami.2c15044. Epub 2022 Oct 27. ACS Appl Mater Interfaces. 2022. PMID: 36302180
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
-
- Xie P., Liu T., Sun J., Yang J.. Structures, properties, and device applications for [1]benzothieno[3,2-b]benzothiophene derivatives. Adv. Funct. Mater. 2022;32(21):2200843. doi: 10.1002/adfm.202200843. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
