Reductive Cleavage of Dioxygen Mediated by a Lewis Superacidic Bis(borane)
- PMID: 40747070
- PMCID: PMC12308418
- DOI: 10.1021/jacsau.5c00516
Reductive Cleavage of Dioxygen Mediated by a Lewis Superacidic Bis(borane)
Abstract
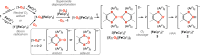
The bis-(borane) o-{(C6F5)2B}2C6F4 (1) reacts with dioxygen in the presence of decamethylferrocene (FeCp*2, Cp* = pentamethylcyclopentadienyl) to deliver the salt [(μ-OH)-o-{B-(C6F5)2}2C6F4]-[FeCp*2] (2) featuring a hydroxide sequestered by the two adjacent boron atoms. Mechanistic investigations of this formal 4-electron reduction of O2 suggest that it goes through the formation of a superoxide adduct [1-O2]-, which evolves through disproportionation into O2 and a peroxo-adduct [1-O2]2-. Upon coordination of another equivalent of 1, the thus-generated 4-fold boron-sequestered peroxide {[1]2-O2}2- undergoes homolytic O-O bond cleavage to yield a pair of oxyl radicals [1-O•]-. These highly reactive intermediates subsequently perform hydrogen atom abstraction to lead to the hydroxide salt 2. Our observations point to FeCp*2 being the H atom purveyor in this final step.
Keywords: Lewis acids; boron; dioxygen; hydrogen atom abstraction; metallocenes; oxyl radical; peroxide; superoxide.
© 2025 The Authors. Published by American Chemical Society.
Figures




References
-
- Oberdorf K., Lichtenberg C.. Small Molecule Activation by Well-Defined Compounds of Heavy p-Block Elements. Chem. Commun. 2023;59(52):8043–8058. doi: 10.1039/D3CC02190D. - DOI - PubMed
- Weetman C.. Main Group Multiple Bonds for Bond Activations and Catalysis. Chem. – Eur. J. 2021;27(6):1941–1954. doi: 10.1002/chem.202002939. - DOI - PMC - PubMed
- Kundu S.. Pincer-Type Ligand-Assisted Catalysis and Small-Molecule Activation by Non-VSEPR Main-Group Compounds. Chem. – Asian J. 2020;15(20):3209–3224. doi: 10.1002/asia.202000800. - DOI - PubMed
- Su Y., Kinjo R.. Small Molecule Activation by Boron-Containing Heterocycles. Chem. Soc. Rev. 2019;48(13):3613–3659. doi: 10.1039/C9CS00072K. - DOI - PubMed
- Wilkins, L. C. ; Melen, R. L. . Small Molecule Activation with Frustrated Lewis Pairs. In Encyclopedia of Inorganic and Bioinorganic Chemistry; John Wiley & Sons, Ltd, 2017; pp 1–24. 10.1002/9781119951438.eibc2520. - DOI
- Yadav S., Saha S., Sen S. S.. Compounds with Low-Valent p-Block Elements for Small Molecule Activation and Catalysis. ChemCatChem. 2016;8(3):486–501. doi: 10.1002/cctc.201501015. - DOI
-
- Slootweg, J. C. ; Jupp, A. R. . Frustrated Lewis Pairs; Springer Nature, 2020.
- Stephan D. W.. The Broadening Reach of Frustrated Lewis Pair Chemistry. Science. 2016;354(6317):aaf7229. doi: 10.1126/science.aaf7229. - DOI - PubMed
- Stephan D. W., Erker G.. Frustrated Lewis Pair Chemistry: Development and Perspectives. Angew. Chem., Int. Ed. 2015;54(22):6400–6441. doi: 10.1002/anie.201409800. - DOI - PubMed
- Frustrated Lewis Pairs I: Uncovering and Understanding; Erker, G. , Stephan, D. W. , Eds.; Topics in Current Chemistry; Springer: Berlin, Heidelberg, 2013.
- Frustrated Lewis Pairs II: Expanding the Scope; Erker, G. , Stephan, D. W. , Eds.; Topics in Current Chemistry; Springer: Berlin, Heidelberg, 2013.
- Stephan D. W., Erker G.. Frustrated Lewis Pairs: Metal-Free Hydrogen Activation and More. Angew. Chem., Int. Ed. 2010;49(1):46–76. doi: 10.1002/anie.200903708. - DOI - PubMed
-
- van der Zee L. J. C., Pahar S., Richards E., Melen R. L., Slootweg J. C.. Insights into Single-Electron-Transfer Processes in Frustrated Lewis Pair Chemistry and Related Donor–Acceptor Systems in Main Group Chemistry. Chem. Rev. 2023;123(15):9653–9675. doi: 10.1021/acs.chemrev.3c00217. - DOI - PMC - PubMed
- Ju M., Lu Z., Novaes L. F. T., Martinez Alvarado J. I., Lin S.. Frustrated Radical Pairs in Organic Synthesis. J. Am. Chem. Soc. 2023;145(36):19478–19489. doi: 10.1021/jacs.3c07070. - DOI - PMC - PubMed
- Dasgupta A., Richards E., Melen R. L.. Frustrated Radical Pairs: Insights from EPR Spectroscopy. Angew. Chem., Int. Ed. 2021;60(1):53–65. doi: 10.1002/anie.202010633. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
