Mechanisms and therapeutics of insulin signaling transduction genes in diabetic cardiomyopathy: a comprehensive updated review
- PMID: 40747301
- PMCID: PMC12310498
- DOI: 10.3389/fendo.2025.1589695
Mechanisms and therapeutics of insulin signaling transduction genes in diabetic cardiomyopathy: a comprehensive updated review
Abstract
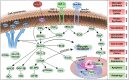
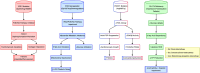
Diabetic cardiomyopathy (DCM), a complication of type 2 diabetes mellitus (T2DM), is closely associated with key genes in the insulin signaling pathway. Insulin regulates cellular metabolism and growth under normal conditions by activating downstream signaling pathways through its receptors. Nonetheless, insulin resistance, which compromises the insulin signaling pathway and impairs cardiovascular system performance, is common in individuals with T2DM. The key insulin signaling genes include IRS1, IRS2, PIK3R1, and GLUT4 play important roles in insulin receptor signaling, PI3K complex assembly, and glucose transport, respectively. Mutations or abnormal expression of these genes may lead to disorders in the insulin signaling pathway, affecting the normal regulation of glucose metabolism and impairment of myocardial function, thereby promoting the development of DCM. This review delves into the specific roles of these genes in the pathogenic mechanisms and treatment of DCM, with the aim of providing scientific evidence and guidance for future research endeavors.
Keywords: diabetic cardiomyopathy; gene; heart failure; insulin signaling; treatment.
Copyright © 2025 He, Yang, He, Wang, Li, Yuan and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Established and Emerging Roles of Epigenetic Regulation in Diabetic Cardiomyopathy.Diabetes Metab Res Rev. 2025 Sep;41(6):e70081. doi: 10.1002/dmrr.70081. Diabetes Metab Res Rev. 2025. PMID: 40831067 Free PMC article. Review.
-
Asprosin attenuates diabetic cardiomyopathy through inhibiting autophagy mediated by AMPK/mTOR/ULK1 pathway.Am J Physiol Cell Physiol. 2025 Aug 1;329(2):C377-C394. doi: 10.1152/ajpcell.01006.2024. Epub 2025 Jun 25. Am J Physiol Cell Physiol. 2025. PMID: 40560772
-
From Type 2 Diabetes Mellitus To Diabetic Cardiomyopathy - A Systematic Review On The Role Of MicroRNA.Curr Diab Rep. 2025 Jul 15;25(1):42. doi: 10.1007/s11892-025-01590-6. Curr Diab Rep. 2025. PMID: 40663215 Free PMC article. Review.
-
Role of rosuvastatin and pitavastatin in alleviating diabetic cardiomyopathy in rats: Targeting of RISK, NF-κB/ NLRP3 inflammasome and TLR4/ NF-κB signaling cascades.PLoS One. 2025 Jul 30;20(7):e0325767. doi: 10.1371/journal.pone.0325767. eCollection 2025. PLoS One. 2025. PMID: 40737341 Free PMC article.
-
Diabetic Cardiomyopathy: An Update on Emerging Pathological Mechanisms.Curr Cardiol Rev. 2025;21(2):e1573403X331870. doi: 10.2174/011573403X331870241025094307. Curr Cardiol Rev. 2025. PMID: 39501954 Review.
References
-
- Seferovic PM, Petrie MC, Filippatos GS, Anker SD, Rosano G, Bauersachs J, et al. Type 2 diabetes mellitus and heart failure: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. (2018) 20:853–72. doi: 10.1002/ejhf.1170, PMID: - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

