Bioinformatic analysis identifies LPL as a critical gene in diabetic kidney disease via lipoprotein metabolism
- PMID: 40747306
- PMCID: PMC12310451
- DOI: 10.3389/fendo.2025.1620032
Bioinformatic analysis identifies LPL as a critical gene in diabetic kidney disease via lipoprotein metabolism
Erratum in
-
Correction: Bioinformatic analysis identifies LPL as a critical gene in diabetic kidney disease via lipoprotein metabolism.Front Endocrinol (Lausanne). 2025 Nov 14;16:1732027. doi: 10.3389/fendo.2025.1732027. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 41323982 Free PMC article.
Abstract
Background: Diabetic kidney disease (DKD) is a common and serious complication of diabetes, affecting approximately 40% of patients with the condition. The pathogenesis of DKD is complex, involving multiple processes such as metabolism, inflammation, and fibrosis. Given its increasing incidence and associated mortality, there is an urgent need to identify novel pathogenic genes and therapeutic targets.
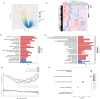
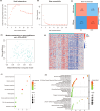
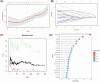
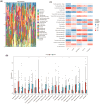
Methods: This study systematically identified hub DKD-associated genes and their potential molecular mechanisms through bioinformatic analysis. Gene expression datasets from DKD patients and healthy controls were obtained from the GEO database. Hub genes were screened using differential expression analysis, weighted gene co-expression network analysis (WGCNA), LASSO regression, random forest (RF) algorithms, and consensus clustering for DKD patient classification. Additionally, immune cell infiltration analysis was performed on differentially expressed genes to explore the relationship between hub genes and the immune microenvironment. Potential drugs targeting LPL were predicted based on gene-drug interaction analysis. Immunohistochemistry was used to verify the expression of LPL and TNF-α in kidney tissues from patients with varying degrees of DKD severity, as well as their relationship with kidney function impairment.
Results: This study revealed that LPL, a lipoprotein metabolism gene, plays a crucial role in DKD, participating in cholesterol and glycerolipid metabolism as well as PPAR signaling. LPL expression was negatively correlated with pro-inflammatory M1 macrophages and various subsets of T cells, including naïve CD4 T cells and gamma delta T cells, while positively correlated with follicular helper T cells, suggesting its immune-regulation effects in DKD progression. Potential LPL-targeting drugs, such as Ibrolipim, anabolic steroid, and acarbose, might mitigate DKD. LPL expression was decreased with DKD severity and was correlated with TNF-α and kidney dysfunction markers, indicating its key role in DKD progression.
Conclusion: LPL is a pivotal regulator of lipid metabolism and immune inflammation in DKD. Potential drugs targeting LPL offer new candidates for precision treatment of DKD. These findings lay a theoretical foundation for understanding the molecular mechanisms of DKD and developing LPL-based therapeutic strategies.
Keywords: bioinformatic; diabetic kidney disease; immune cell infiltration; lipid metabolism; lipoprotein lipase.
Copyright © 2025 Dong, Xu, Xu, Liu and Shen.
Conflict of interest statement
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Fermín-Martínez CA, Bello-Chavolla OY, Paz-Cabrera CD, Ramírez-García D, Perezalonso-Espinosa J, Fernández-Chirino L, et al. Prediabetes as a risk factor for all-cause and cause-specific mortality: a prospective analysis of 115,919 adults without diabetes in Mexico City. MedRxiv Prepr Serv Health Sci. (2024), 2024.04.15.24305840. doi: 10.1101/2024.04.15.24305840, PMID: - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

