Sequence, characterization and pharmacological analyses of the adipokinetic hormone receptor in the stick insect, Carausius morosus
- PMID: 40747312
- PMCID: PMC12310481
- DOI: 10.3389/fendo.2025.1601334
Sequence, characterization and pharmacological analyses of the adipokinetic hormone receptor in the stick insect, Carausius morosus
Abstract
Background: Adipokinetic/hypertrehalosaemic hormone (AKH/HrTH), corazonin (Crz) and the AKH/Crz-related peptide (ACP) are neuropeptides considered homologous to the vertebrate gonadotropin-releasing hormone (GnRH). AKH/HrTH are important peptidergic metabolic regulators in insects that are crucial to provide energy during periods of high output mobility or when large amounts of energy-rich substrates are synthesized (for example, during vitellogenesis). AKH functions via a G protein-coupled receptor. Understanding which residue of the peptide (the ligand), activates the receptor with high efficacy is an important step to get insights into the ligand-receptor interaction, which is essential for further research on creating a model of how the ligand behaves in the binding pocket of the receptor. Such data are necessary for the search of non-peptidic mimetic agonists or antagonists in pesticide design.
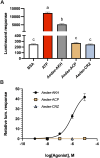
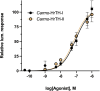
Methods: Using bioinformatics and cloning techniques, the complete coding sequence of an AKH receptor was cloned and sequenced from fat body tissues and nervous tissues from the Indian stick insect, Carausius morosus. The resulting Carmo-AKHR was then expressed in a mammalian cell line where it could couple with a Gq protein to mediate calcium mobilization in vitro and cause bioluminescence when activated by a ligand. This receptor assay was used not only with the natural AKH ligands of the stick insect, but also with AKHs from other species and analogs with targeted modifications. A phylogenetic analysis of Carmo-AKHR with the AKH receptors and related receptors from other insects was also carried out.
Results: The stick insect AKH receptor was successfully cloned and sequenced from fat body and, separately, from nervous tissues. Comparison with known insect AKH, Crz and ACP receptors clearly put the stick insect receptor in the AKH clade and as sister group to other putative Phasmatodean AKH receptors. Moreover, the receptor expressed in mammalian cells was only activated by AKH and not by Crz or ACP indicating a true AKH receptor. Structure-activity studies in an Ala replacement series revealed the ligand residues that are absolutely essential for activating the AKHR: the N-terminal pGlu, Phe4, Trp8 and the C-terminal carboxyamide. Almost as important are Thr3 and Thr5 since their replacement reduced the efficacy more than a 100-fold, whereas Thr10 can be replaced without any real loss of activity. When substituted by Ala at positions 2, 6, 7 and 9, the ligand is somewhat affected with the loss of receptor activation being between 5- to 20-fold. Chain length of the ligand is important for the receptor: an octa- or nonapeptide with the same sequence otherwise as the endogenous stick insect ligand, display a 5- to 10 fold reduced activity. Carefully selected naturally occurring AKH analogs from other insects support the above results.
Conclusions: The AKH receptor from stick insects (Phasmatodea) cluster together in one clade distinct from other insect AKHRs, although still similar enough to be an insect AKHR, as opposed to the other GnRH-related receptors of insects, such as ACP and Crz receptors. The phylogenetic analyses support the data obtained from other studies involving receptors for AKH, Crz and ACP peptides. The receptor assay results with AKH analogs corroborated most of the results obtained previously using in vivo studies, thus emphasizing that the endogenous AKHs operate through this receptor to cause hypertrehalosemia in the stick insect. It is also clear that certain residues of the AKH peptides are consistently important in their interaction with the cognate AKH receptor, while other amino acid residues are of different importance to AKH receptors on a broad species- or group-specific manner. The previously observed peculiarity that hypertrehalosemia, in response to AKH injection, is only measurable in stick insects ligated below the head is discussed. No explanations for this, however, can be inferred from the current study.
Keywords: G protein-coupled receptor; adipokinetic hormone receptor; alanine replacement series; in vitro receptor activation; metabolism; pharmacological analyses; stick insect; structure-activity relationship.
Copyright © 2025 Gäde, Tan, Afifi, Paluzzi, Jackson and Marco.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
The Adipokinetic Hormone (AKH) and the Adipokinetic Hormone/Corazonin-Related Peptide (ACP) Signalling Systems of the Yellow Fever Mosquito Aedes aegypti: Chemical Models of Binding.Biomolecules. 2024 Mar 6;14(3):313. doi: 10.3390/biom14030313. Biomolecules. 2024. PMID: 38540733 Free PMC article.
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
References
-
- Liessem S, Kowatschew D, Dippel S, Blanke A, Korsching S, Guschlbauer C, et al. Neuromodulation can be simple: Myoinhibitory peptide, contained in dedicated regulatory pathways is the only neurally-mediated peptide modulator of stick insect leg muscle. J Neurosci. (2021) 41:2911–29. doi: 10.1523/JNEUROSCI.0188-20.2021, PMID: - DOI - PMC - PubMed
-
- Gäde G. Adipokinetic and hyperglycaemic factor(s) in the corpora cardiaca/corpora allata complex of the stick insect, Carausius morosus. I. Initial characteristics. Physiol Entomol. (1979) 4:131–4.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

