Loss of olfaction reduces caterpillar performance and increases susceptibility to a natural enemy
- PMID: 40747879
- PMCID: PMC12316457
- DOI: 10.7554/eLife.105585
Loss of olfaction reduces caterpillar performance and increases susceptibility to a natural enemy
Abstract
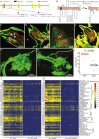
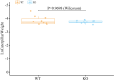
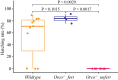
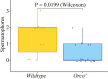
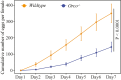
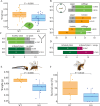
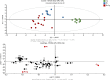
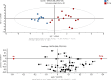
Insect herbivores such as caterpillars are under strong selection pressure from natural enemies, especially parasitoid wasps. Although the role of olfaction in host-plant seeking has been investigated in great detail in parasitoids and adult lepidopterans, the caterpillar olfactory system and its significance in tri-trophic interactions remain poorly understood. In this study, we investigated the olfactory system of Pieris brassicae caterpillars and the importance of olfactory information in the interactions among this herbivore, its host-plant Brassica oleracea, and its primary natural enemy Cotesia glomerata. To examine the role of olfaction, we utilized CRISPR/Cas9 to knockout (KO) the odorant receptor co-receptor (Orco). This KO impaired olfactory detection and primary processing in the brain. Orco KO caterpillars exhibited reduced weight and lost preference for their optimal food plants. Interestingly, the KO caterpillars also experienced reduced weight when challenged by the parasitoid C. glomerata whose ovipositor had been removed, and the mortality of the KO caterpillars under the attack of unmanipulated parasitoids increased. We then investigated the behavior of P. brassicae caterpillars in response to volatiles from plants attacked by conspecific caterpillars and volatiles from plants on which the caterpillars were themselves attacked by C. glomerata. After analyzing the volatile compounds involved in these interactions, we concluded that olfactory information enables caterpillars to locate suitable food sources more efficiently as well as to select enemy-free spaces. Our results reveal the crucial role of olfaction in caterpillar feeding and natural-enemy avoidance, highlighting the significance of chemoreceptor genes in shaping ecological interactions.
Keywords: Orco; ecology; host-plant selection; insect larvae; multitrophic interactions; natural enemy; olfaction.
Plain language summary
Many caterpillars are major pests in agriculture, feeding on a variety of crops. They constantly face threats from both predators and the toxic defenses of the plants they eat. While scientists have long studied how predators find their prey, much less is known about how prey – like caterpillars – manage to survive and defend themselves. Plants have evolved various strategies to fend off insects. When attacked by herbivores, some plants release specific chemical signals known as herbivore-induced plant volatiles. These scents act as a distress call, attracting natural enemies of the herbivores, such as parasitoid wasps, which use the caterpillars for reproduction, killing the herbivores in the process. In turn, caterpillars have evolved different defense strategies. They have a sophisticated sense of smell, which may help them detect not only the scent of host plants but also the presence of predators or other caterpillars nearby. Despite how common and important caterpillars are in farming systems, it is still not fully understood how they choose where to feed or how they avoid being eaten. To explore this, Wang et al. studied how much caterpillars rely on their sense of smell to survive and find food. In insects, the ability to smell relies on a group of sensory proteins, most of which need a key gene called Orco to function. Without this gene, an insect’s ability to smell is severely impaired. Wang et al. used a gene editing tool known as CRISPR/Cas9 to ‘turn off’ the Orco gene in caterpillars of the large cabbage white butterfly (Pieris brassicae), effectively disabling their sense of smell. The researchers then compared the development, survival performance, and behavioral preferences of these mutant caterpillars with normal ones. This revealed that caterpillars without a working sense of smell gained less weight and were less successful at finding suitable food sources. They were also more likely to be killed by parasitoid wasps. Behavioral experiments showed that caterpillars with an intact Orco gene avoided plants where other caterpillars were under attack by detecting the warning signals in the air. In contrast, mutant caterpillars could not recognize these danger cues and were unable to find suitable host plants or select enemy-free spaces. The study of Wang et al. shows that caterpillars use smell not only to find food, but also to avoid danger. These insights could help farmers develop new, environmentally friendly ways to manage pests. For example, by planting companion plants that produce specific scents, or by breeding crops that naturally repel pests, we might be able to steer caterpillars away from valuable crops without relying on chemical pesticides.
© 2025, Wang et al.
Conflict of interest statement
QW, YJ, HS, BW, LG, MJ, MD, AH No competing interests declared
Figures





Update of
- doi: 10.1101/2024.12.17.629055
- doi: 10.7554/eLife.105585.1
- doi: 10.7554/eLife.105585.2
Similar articles
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
"In a State of Flow": A Qualitative Examination of Autistic Adults' Phenomenological Experiences of Task Immersion.Autism Adulthood. 2024 Sep 16;6(3):362-373. doi: 10.1089/aut.2023.0032. eCollection 2024 Sep. Autism Adulthood. 2024. PMID: 39371355
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
-
- Bernays EA. Feeding by lepidopteran larvae is dangerous. Ecological Entomology. 1997;22:121–123. doi: 10.1046/j.1365-2311.1997.00042.x. - DOI
-
- Bourne ME, Gloder G, Weldegergis BT, Slingerland M, Ceribelli A, Crauwels S, Lievens B, Jacquemyn H, Dicke M, Poelman EH. Parasitism causes changes in caterpillar odours and associated bacterial communities with consequences for host-location by a hyperparasitoid. PLOS Pathogens. 2023;19:e1011262. doi: 10.1371/journal.ppat.1011262. - DOI - PMC - PubMed
-
- Brodeur J, Geervliet JBF, Vet LEM. The role of host species, age and defensive behaviour on ovipositional decisions in a solitary specialist and gregarious generalist parasitoid (Cotesia species) Entomologia Experimentalis et Applicata. 1996;81:125–132. doi: 10.1111/j.1570-7458.1996.tb02024.x. - DOI
-
- Carpenter JE, Sparks AN. Effects of vision on mating behavior of the male corn earworm. Journal of Economic Entomology. 1982;75:248–250. doi: 10.1093/jee/75.2.248. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

