Development of the First Small-Molecule Inhibitor Targeting Oncostatin M for Treatment of Breast Cancer
- PMID: 40747931
- PMCID: PMC12362618
- DOI: 10.1021/acs.jmedchem.4c03233
Development of the First Small-Molecule Inhibitor Targeting Oncostatin M for Treatment of Breast Cancer
Abstract
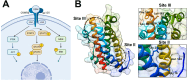
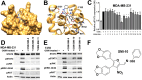
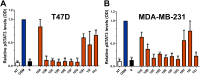
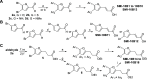
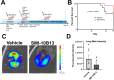
Oncostatin M (OSM) is a proinflammatory cytokine implicated in inflammatory diseases and multiple cancers, especially breast cancer. To date, no federally approved anti-OSM therapeutics exist. We computationally screened ∼1.65 million compounds to identify small-molecule inhibitors (SMIs) of the OSM, and candidates were validated in human breast cancer models. We identified a tetrasubstituted furan (SMI-10) that inhibited OSM signaling, and optimization generated SMI-10B (KD = 12.9 μM) and SMI-10B13 (KD = 6.6 μM). SMI-10B13 strongly inhibited OSM-mediated STAT3 phosphorylation in T47D and MCF-7 cell lines (IC50= 136 and 164 nM, respectively). Fluorescence quenching, NMR, and surface plasmon resonance assays were used to characterize SMI/OSM interactions and identify a number of analogs with low-micromolar affinity for OSM. In a human breast cancer mouse model, SMI-10B13 reduced tumor growth (p < 0.001). Kaplan-Meier analysis showed improved survival in SMI-10B13-treated mice (p = 0.04), highlighting its potential as the first anti-OSM therapeutic to inhibit breast cancer progression and extend survival.
Figures
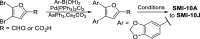




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

