COVID-19 vaccination around the time of conception and risk of placenta-mediated adverse pregnancy outcomes
- PMID: 40747952
- PMCID: PMC12451205
- DOI: 10.1111/aogs.70025
COVID-19 vaccination around the time of conception and risk of placenta-mediated adverse pregnancy outcomes
Abstract
Introduction: Although numerous studies have documented no association between COVID-19 vaccination during pregnancy and maternal and fetal health outcomes, fewer studies have evaluated fetal health effects after COVID-19 vaccination around the time of conception and early pregnancy, a time when maternal exposures may affect early placentation and the subsequent risk of placenta-mediated adverse pregnancy outcomes.
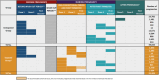
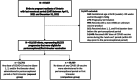
Material and methods: We used province-wide databases in Ontario to conduct a population-based cohort study including all live and stillbirths ≥20 weeks' gestation with a last menstrual period (LMP) between April 1 and December 31, 2021. We deterministically linked birth registry data to the vaccine registry for all 80 253 eligible pregnancies; 31 209 (38.9%) received ≥1 dose of the COVID-19 vaccine around the time of conception or first trimester. Using Cox regression, we estimated propensity score weighted hazard ratios (aHR) and 95% confidence intervals (CI) for associations between ≥1 dose of mRNA COVID-19 vaccine during the periconceptional/first trimester exposure window (28 days before the LMP to the end of first trimester) and study outcomes: hypertensive disorders (gestational hypertension, preeclampsia, eclampsia), placental abruption, preterm birth (<37 weeks), small-for-gestational-age (SGA) birth (<10th percentile), and stillbirth.
Results: COVID-19 vaccination around the time of conception or first trimester was associated with a small increased risk of hypertensive disorders in pregnancy in exposed versus unexposed individuals (7.4% vs. 6.1%; aHR: 1.10, 95% CI: 1.03-1.17), mostly attributed to gestational hypertension (5.0% vs. 4.1%; aHR 1.13, 95% CI: 1.05-1.22). There was no increased risk of preeclampsia (1.8% vs. 1.5%; aHR 1.08, 95% CI: 0.95-1.22), eclampsia (0.1% vs. 0.1%; aHR: 1.12, 95% CI 0.65-1.95), placental abruption (0.8% vs. 1.0%; aHR: 0.77, 95%CI: 0.65-0.91), preterm birth (8.0% vs. 8.9%; aHR: 0.92, 95%CI: 0.87-0.97), SGA birth (8.9% vs. 9.3%; aHR: 1.00, 95%CI: 0.95-1.06), or stillbirth (0.4% vs. 0.6%; aHR: 0.66, 95%CI: 0.52-0.82).
Conclusions: This population-based Canadian study provides additional evidence evaluating COVID-19 vaccine administration around the start of pregnancy. While we identified no association with most placenta-mediated outcomes, we report a slight increase in the rate of gestational hypertension. This could be a true association or attributed to residual confounding. Further research is needed to verify.
Keywords: COVID‐19 vaccines; first; placenta; pregnancy; pregnancy trimester.
© 2025 The Author(s). Acta Obstetricia et Gynecologica Scandinavica published by John Wiley & Sons Ltd on behalf of Nordic Federation of Societies of Obstetrics and Gynecology (NFOG).
Conflict of interest statement
Dr. Regan reported receiving grants from the National Institutes of Health/National Institute of Allergy and Infectious Diseases and the EuroQol Research Foundation for research unrelated to the submitted work and membership on a Moderna Data Safety Monitoring Board. When this project was funded and initiated, Dr. Fell was employed by the University of Ottawa and had an academic appointment at the Children's Hospital of Eastern Ontario Research Institute; although she maintains adjunct appointments at both institutions, she is now employed by Pfizer and works on an unrelated topic. Dr. K. Wilson reported being CEO of CANImmunize Inc. and being on advisory boards for Medicago and Moderna. No other disclosures were reported.
Figures

References
-
- Government of Canada . COVID‐19 vaccines: Canadian immunization guide. Accessed October 11, 2024. https://www.canada.ca/en/public‐health/services/publications/healthy‐liv...
-
- Lapinsky SC, Baxter NN, Sutradhar R, et al. A population‐based test‐negative matched case‐control analysis of SARS‐CoV‐2 vaccine effectiveness among pregnant people in Ontario, Canada. J Obstet Gynaecol Can. 2024;46:102239. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

