Conserved and unique features of terminal telomeric sequences in ALT-positive cancer cells
- PMID: 40748043
- PMCID: PMC12316455
- DOI: 10.7554/eLife.106657
Conserved and unique features of terminal telomeric sequences in ALT-positive cancer cells
Abstract
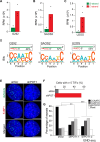
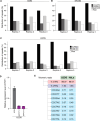
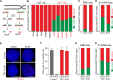
A significant portion of human cancers utilize a recombination-based pathway, alternative lengthening of telomeres (ALT), to maintain telomere length. Targeting the ALT is of growing interest as a cancer therapy, yet a substantial knowledge gap remains regarding the basic features of telomeres in ALT-positive cells. To address this, we adopted END-seq, an unbiased sequencing-based approach, to define the identity and regulation of the terminal sequences of chromosomes in ALT cells. Our data reveal that the terminal portions of chromosomes in ALT cells contain canonical telomeric sequences with the same terminus bias (-ATC) observed in non-ALT cells. Furthermore, as reported for non-ALT cells, POT1 is required to preserve the precise regulation of the 5' end in cells that maintain telomere length using the ALT pathway. Thus, the regulation of the terminal 5' of chromosomes occurs independently of the mechanism of telomere elongation. Additionally, we employed an S1 endonuclease-based sequencing method to determine the presence and origin of single-stranded regions within ALT telomeres. These data shed light on conserved and unique features of ALT telomeres.
Keywords: ALT; END-seq; cancer biology; chromosomes; gene expression; human; mouse; ssDNA; telomeres.
Conflict of interest statement
BA, WW, RP, RS, TM, AN, EL No competing interests declared
Figures





Update of
-
Conserved and Unique Features of Terminal Telomeric Sequences in ALT-Positive Cancer Cells.bioRxiv [Preprint]. 2025 Jun 2:2025.02.27.640565. doi: 10.1101/2025.02.27.640565. bioRxiv. 2025. Update in: Elife. 2025 Aug 01;14:RP106657. doi: 10.7554/eLife.106657. PMID: 40501549 Free PMC article. Updated. Preprint.
References
-
- Azeroglu B, Khurana S, Wang SC, Tricola GM, Sharma S, Jubelin C, Cortolezzis Y, Pegoraro G, Miller KM, Stracker TH, Lazzerini Denchi E. Identification of modulators of the ALT pathway through a native FISH-based optical screen. Cell Reports. 2025;44:115114. doi: 10.1016/j.celrep.2024.115114. - DOI - PMC - PubMed
-
- Barroso-González J, García-Expósito L, Hoang SM, Lynskey ML, Roncaioli JL, Ghosh A, Wallace CT, de Vitis M, Modesti M, Bernstein KA, Sarkar SN, Watkins SC, O’Sullivan RJ. RAD51AP1 is an essential mediator of alternative lengthening of telomeres. Molecular Cell. 2020;79:202006026. doi: 10.1016/j.molcel.2020.06.026. - DOI - PMC - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

