Meteorins regulate the formation of the left-right organizer and the establishment of vertebrate body asymmetry
- PMID: 40748055
- PMCID: PMC12316458
- DOI: 10.7554/eLife.105430
Meteorins regulate the formation of the left-right organizer and the establishment of vertebrate body asymmetry
Abstract
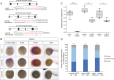
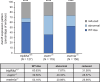
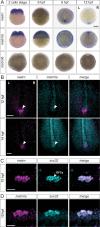
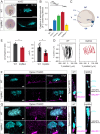
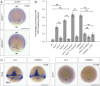
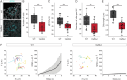
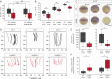
While the exterior of vertebrate bodies appears bilaterally symmetrical, internal organ positioning and morphology frequently exhibit left-right (L-R) asymmetries. In several vertebrates, including human, mouse, frog, and zebrafish, left-right symmetry-breaking during embryonic development is initiated by a ciliated organ called the Node or left-right organizer. Within the Node, a leftward flow of extraembryonic fluid named the Nodal flow mediates the asymmetric expressions of Nodal factors. Although downstream Nodal pathway components leading to the establishment of the embryonic left-right axis are well known, less is known about the development and formation of the embryonic Node itself. Here, we reveal a novel role for the Meteorin protein family in the establishment of the left-right axis and in the formation of Kupffer's vesicle, the Node equivalent structure in zebrafish. We show that the genetic inactivation of each or all three members of the zebrafish Meteorin family (metrn, metrn-like a, and metrn-like b) leads to defects in properties of the Kupffer's vesicle, caused by impaired assembly and migration of the Kupffer's vesicle forming dorsal forerunner cells. In addition, we demonstrate that Meteorins genetically interact with integrins ItgαV and Itgβ1b, regulating the dorsal forerunner cell clustering, and that meteorins loss-of-function results in disturbed Nodal factor expression and consequently in randomized or symmetric heart looping and jogging. These results identify a new role for the Meteorin protein family in the left-right asymmetry patterning during embryonic vertebrate development.
Keywords: Meteorin; developmental biology; embryonic development; left-right asymmetry; zebrafish.
© 2025, Eggeler et al.
Conflict of interest statement
FE, JB, LB, KD, TA, SA No competing interests declared, FD is affiliated with ZeClinics SL. The author has no other competing interests to declare, FD Reviewing editor, eLife
Figures






Update of
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

