Targeting Src tyrosine kinase to enhance radioiodide uptake in breast cancer
- PMID: 40748136
- PMCID: PMC12372067
- DOI: 10.1530/ERC-24-0312
Targeting Src tyrosine kinase to enhance radioiodide uptake in breast cancer
Abstract
Sodium iodide symporter (NIS) expression in breast cancer renders radioiodide (RAI) a promising treatment modality. However, insufficient functional NIS within the plasma membrane limits RAI uptake (RAIU). We aimed to elucidate NIS regulatory mechanisms that impede RAIU in breast cancer and identify molecular targets for stimulating RAI-avidity in breast tumours. Mechanistic interaction between pituitary tumor-transforming gene-binding factor (PBF/PTTG1IP) and NIS was investigated through NanoBiT, co-immunoprecipitation, immunofluorescent microscopy, subcellular localisation and RAIU assays utilising wild-type and CRISPR-Cas9 PBF knockout breast cancer cells. In breast cancer cells, NIS:PBF interaction resulted in diminished RAIU, reversible through reduced PBF phosphorylation by the Src inhibitor dasatinib. Src overexpression diminished RAIU in a PBF-dependent manner that was mediated by Src myristoylation by N-myristoyltransferase 1 (NMT1). NMT1 inhibition significantly enhanced RAIU via Src and PBF in breast and thyroid cancer cells. Bioinformatic analyses revealed clinical associations between high Src and NMT1 expression and increased tumour recurrence in RAI-treated thyroid cancers indicating RAI-resistance. In breast cancer, high PBF and Src expression was associated with the more aggressive tumours that are most likely to benefit from targeted RAI therapy. We describe a new NIS regulatory pathway in breast cancer cells via Src myristoylation and PBF phosphorylation and show that the same pathway exists in thyroid cells, the canonical setting for the exploitation of NIS function. These findings reveal that PBF interaction with NIS may be modulated by Src, which in turn is susceptible to NMT inhibition, and suggest that targeting NMT1 may represent an innovative approach for augmenting RAI-avidity in breast cancer.
Keywords: NIS; PBF/PTTG1IP; Src myristoylation; breast cancer; radioiodide.
Conflict of interest statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the work reported.
Figures
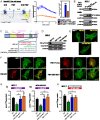
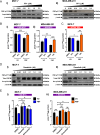
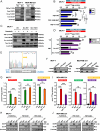

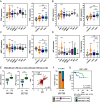
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

